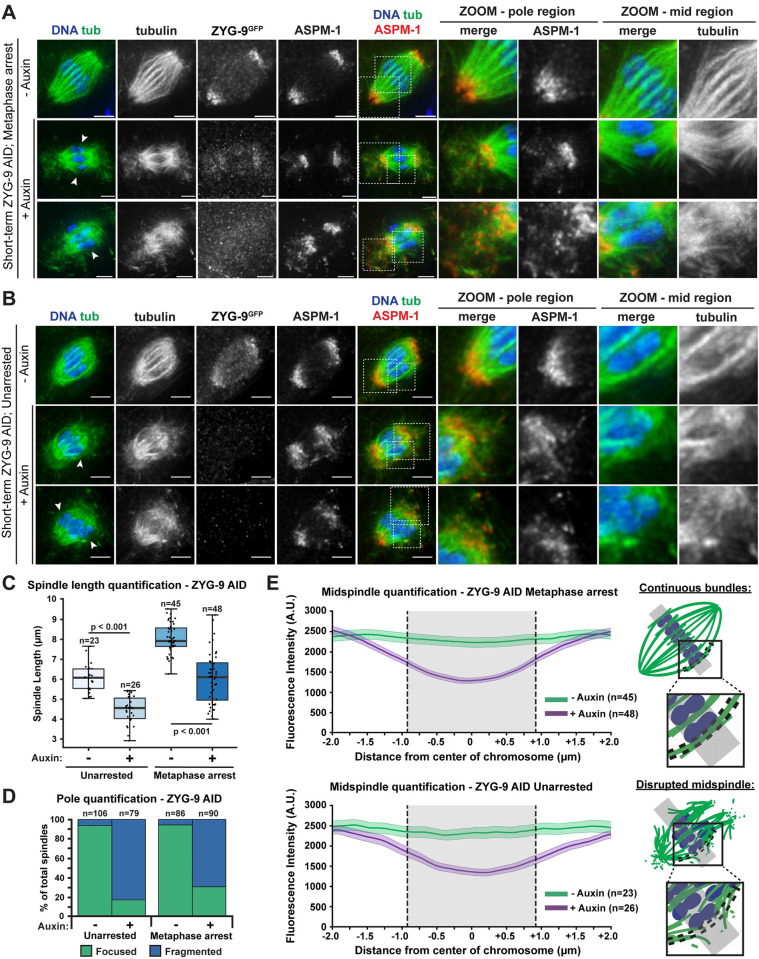
Fig 3. ZYG-9 depletion disrupts pole organization, midspindle bundle stability, and spindle length.
(A, B) Spindles from Metaphase I-arrested (emb-30(RNAi)) oocytes (A) or unarrested oocytes (B) treated with vehicle or 1mM auxin for 25–30 minutes. Shown are DNA (blue), tubulin (green), ASPM-1 (red), and ZYG-9 (stained with a GFP antibody; not shown in merge). In vehicle-treated oocyte spindles, the poles are tightly organized, and the microtubule bundles appear to cross from one side of the spindle to the other without interruption. In spindles from auxin-treated oocytes, the ZYG-9 signal is decreased and spindle pole integrity is compromised as shown by ASPM-1 and tubulin coming off the poles away from the spindle (zooms–pole region). Spindles from auxin-treated oocytes also showed defects in midspindle microtubules, where they appeared to terminate near the center of the spindle and splay away from the chromosomes (arrowheads, zooms–mid region). Bars = 2.5 μm. (C) Quantification of spindle length (pole to pole) in unarrested and Metaphase I-arrested oocytes treated with either vehicle or 1mM auxin. Box represents the first quartile, median, and third quartile. Whiskers extend to maxima and minima. Significance determined using a two-tailed t-test. n represents the number of spindles analyzed. (D, E) Quantification of pole phenotypes (D) or midspindle bundle splaying (E) from unarrested (vector control) and Metaphase I-arrested (emb-30(RNAi)) oocytes treated with either vehicle or auxin. Schematic in (E) shows how midspindle defects were quantified. Shaded regions of lines represent +/- SEM, and gray shading represents average length of chromosome in that condition. n represents the number of spindles analyzed.