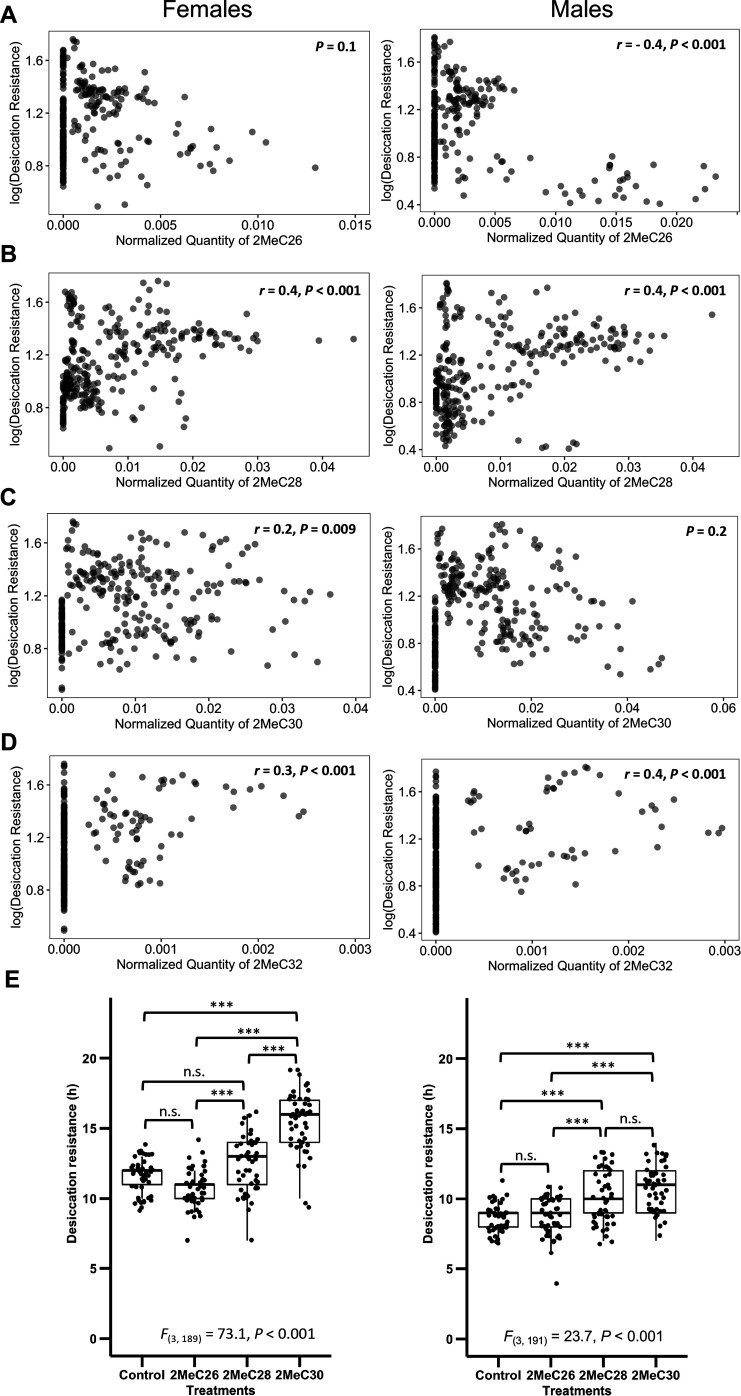
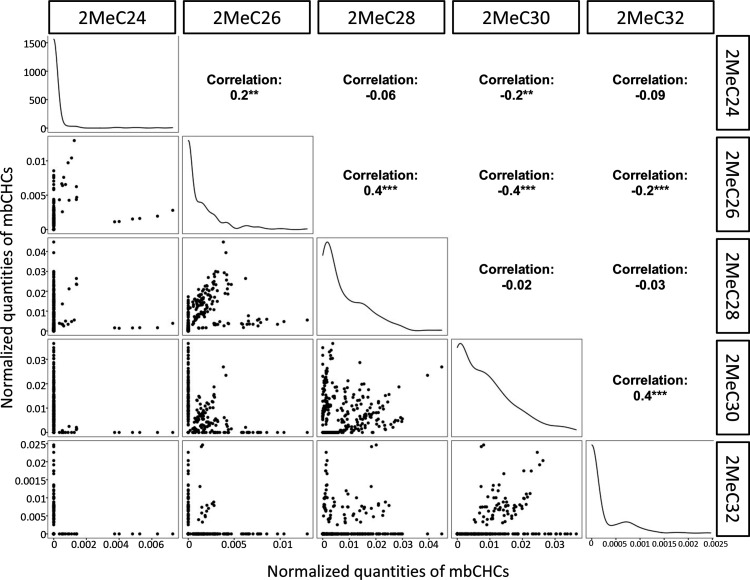
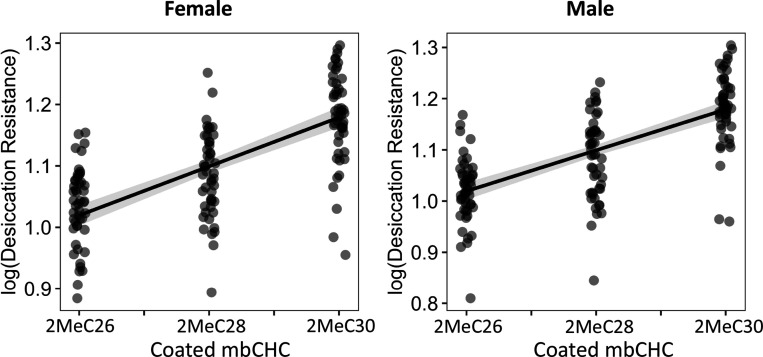
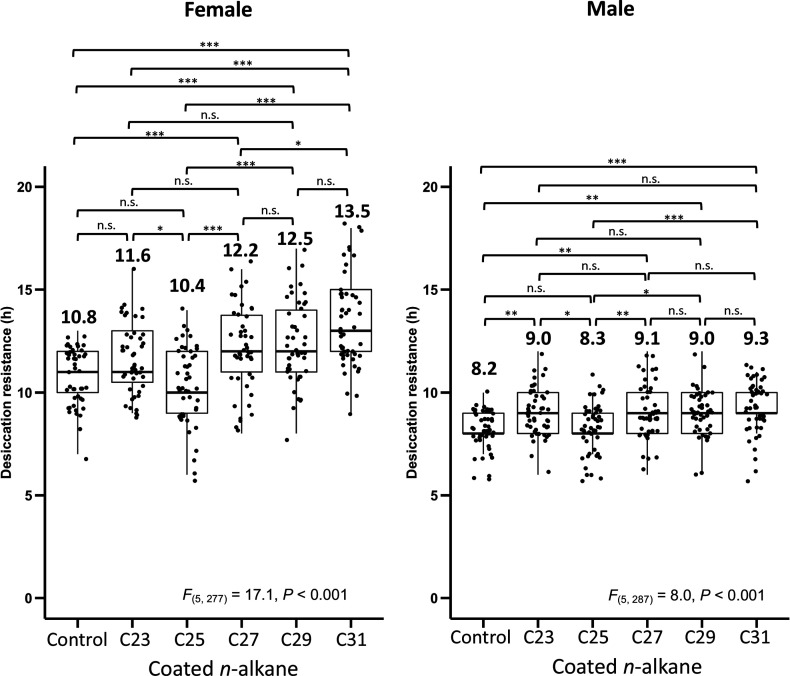
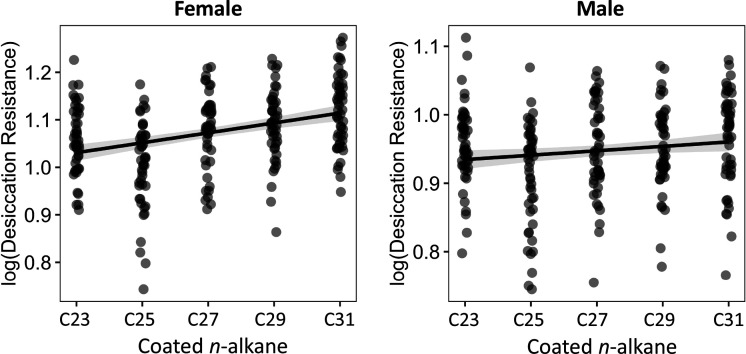
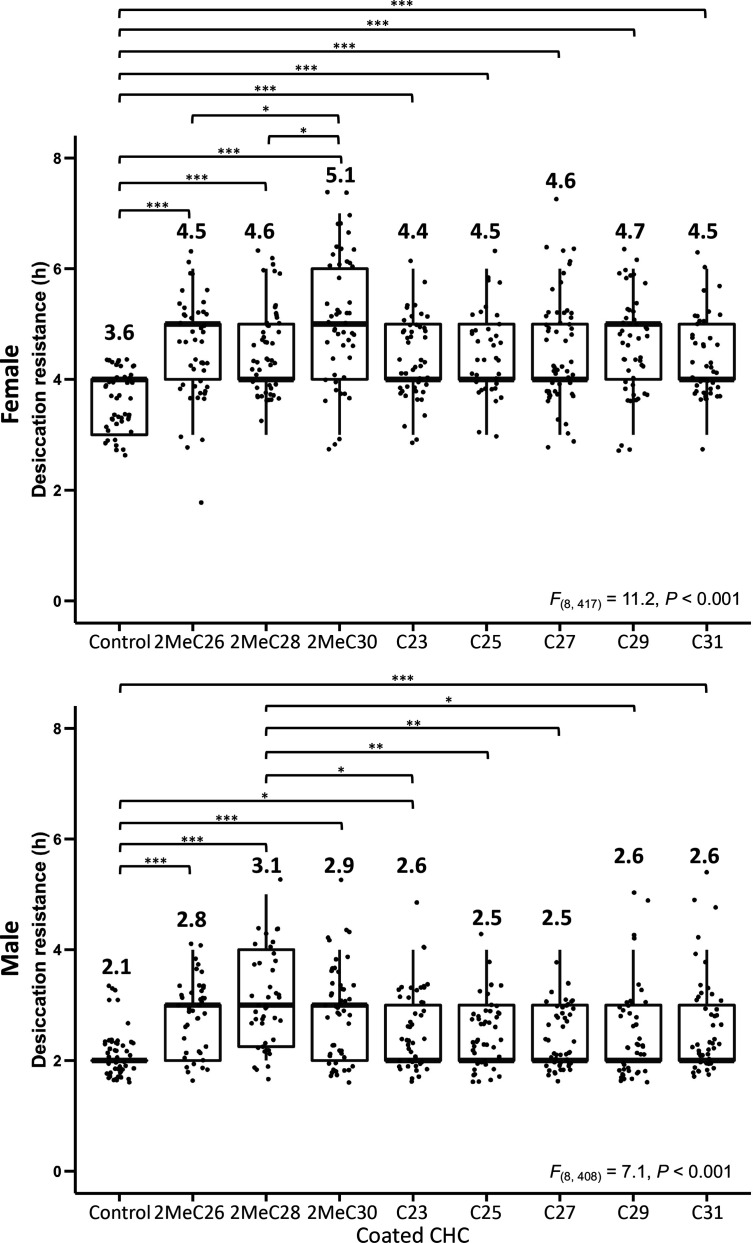
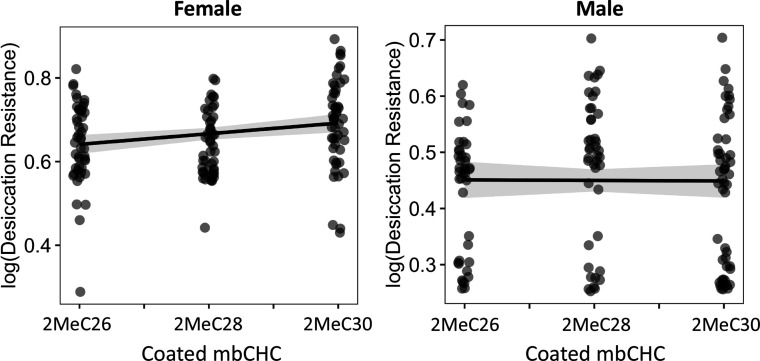
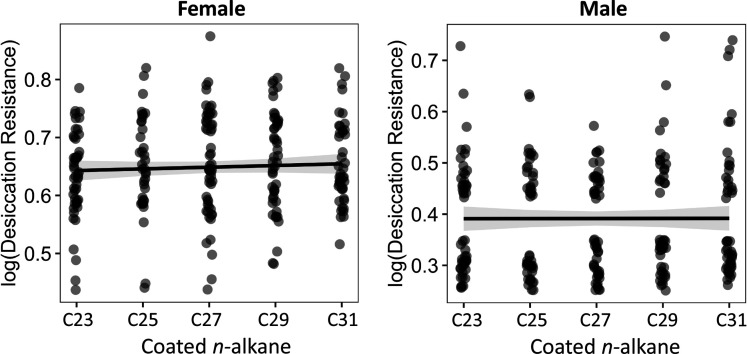
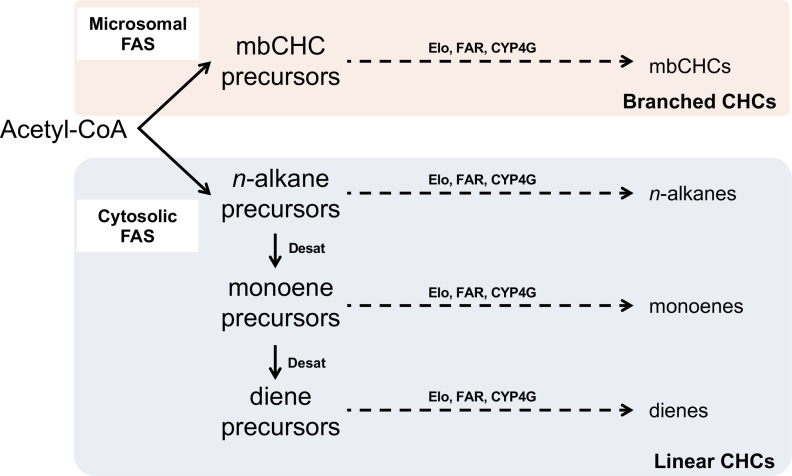
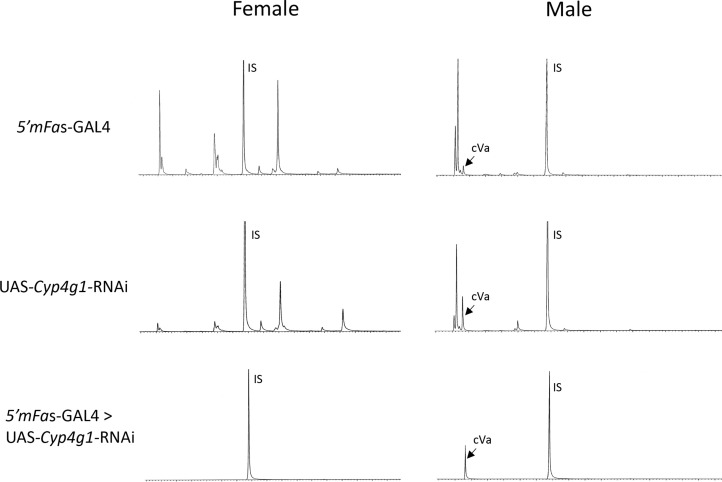
Figure 5. Longer mbCHCs are associated with higher desiccation resistance.
(A) Quantity of 2MeC26 is negatively correlated with desiccation resistance in males, but no correlation in females (Females: p = 0.1, Males: r = −0.4, p < 0.001). (B) Quantity of 2MeC28 is positively correlated with desiccation resistance (Females: r = 0.4, p < 0.001, Males: r = 0.4, p < 0.001). (C) Quantity of 2MeC30 is positively correlated with desiccation resistance in females, but no correlation in males (Females: r = 0.2, p = 0.009, Males: p = 0.2). (D) Quantity of 2MeC32 is positively correlated with desiccation resistance (Females: r = 0.3, p < 0.001, Males: r = 0.4, p < 0.001). Correlations between the quantities of each mbCHC and desiccation resistance were determined using Pearson’s method. (E) D. melanogaster coated with longer mbCHCs have higher desiccation resistance. Coating of synthetic mbCHCs on D. melanogaster showed that 2MeC26 does not influence desiccation resistance. 2MeC30 increases desiccation resistance in female D. melanogaster while both 2MeC28 and 2MeC30 in increases desiccation resistance in male D. melanogaster. The bold horizontal line within each box plot corresponds to the median value, the box length to the interquartile range, and the lines emanating from the box (whiskers) extend to the smallest and largest observations. One-way analysis of variance (ANOVA) showed significant differences between D. melanogaster flies coated with different mbCHCs (Female: F(3,189) = 73.1, p < 0.001, Male: F(3,191) = 23.7, p < 0.001). Post hoc comparison was conducted using Tukey’s method. ***p < 0.001; n.s. refers to not significant.