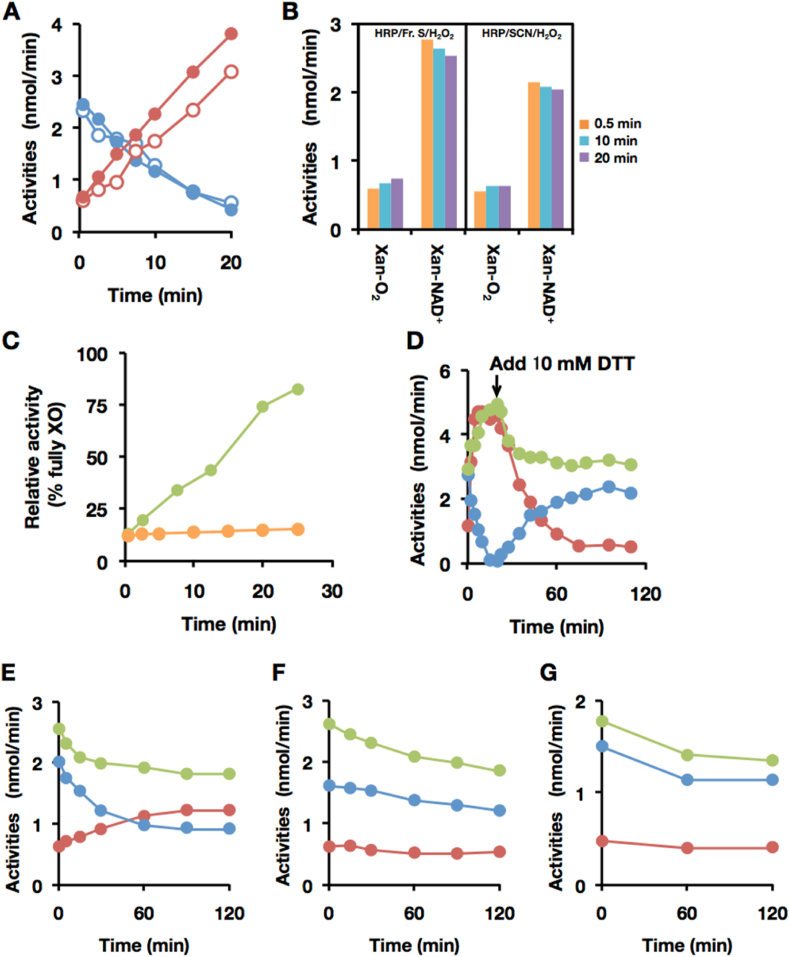
Fig. 5.
Characterization of the D→O factor in Fraction S. (A–C) Effect of thiocyanate on peroxidase-catalyzed D→O activity, using LPO and HRP. The experimental procedures and conditions are the same as described in Fig. 2 other than LPO, HRP and NaSCN were used instead of milk fraction. (A) D→O reaction with LPO/Fraction S/H2O2 (filled circles), or LPO/SCN−/H2O2 (open circles). Red circles, O2-dependent urate formation for XO activity; blue circles, NAD+-dependent NADH formation for XDH activity. (B) D→O reaction with HRP/Fraction S/H2O2(left panel), or HRP/SCN−/H2O2(right panel). O2-dependent urate formation for XO activity (Xan-O2) and NAD+-dependent NADH formation for XDH activity (Xan-NAD+) were assayed at indicated time. (C) Effect of SCNase treatment of Fraction S on D→O activity. Bovine milk XDH was incubated with LPO/Fraction S/H2O2 (green circles), or LPO/SCNase treated Fraction S/H2O2 (orange circles). (D–G) LPO-catalyzed D→O of rat XOR variants. The experimental procedures and conditions are the same as described above except for the rat XOR variants. Red circles: O2-dependent urate formation, blue circles: NAD+-dependent NADH formation, and green circles: NAD + plus O2-dependent urate formation. (D) 2 μM native-XDH (from rat liver, AFR 146, D/O 8.06), (E) 1 μM Rat liver XDH C535A/C992R mutant (AFR 129, D/O 7.42), (F) 1 μM Rat liver XDH C535A/C992R/C1316S mutant (AFR 106.4, D/O 5.06), or (G) 0.5 μM Rat liver XDH C535A/C992R/C1324S mutant (AFR 98.7, D/O 9.125), were incubated with LPO/NaSCN/H2O2. The arrow shows the time at which DTT (10 mM, final) was added in experiment D. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)