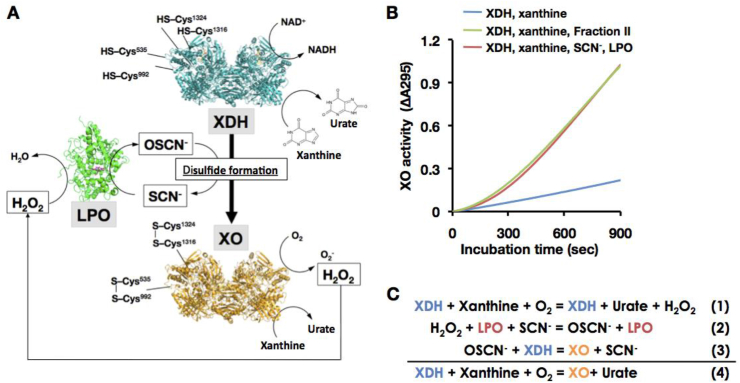
Fig. 6.
Physiological involvement of LPO/SCN/H2O2system to the D→O reaction. (A) The Illustration for D→O activity in bovine milk. Enzyme structures are designed from bovine lactoperoxidase (LPO, PDB ID: 3NYH) [70] and rat xanthine oxidase (XO, PDB ID: 4YTZ) [12], and rat XOR C535A, C992R, and C1324S triple valiants (XDH, PDB ID: 1WYG) [26] are used here. Those corresponding residues are all conserved within mammalian XOR. Cysteine residues (Cys535, Cys992, Cys1316, and Cys1324) responsible for disulfide bridge formation were depicted on the protein structures. When a part of XDH is converted to XO, H2O2 is generated from XO in the presence of xanthine. The generated H2O2 will then participate in the LPO/H2O2/SCN− reaction, resulting in disulfide bond formation and conversion of the dehydrogenase to the oxidase. D→O may well be accelerated synergistically/autocatalytically, also in vivo. (B) Synergistic D→O. Reaction was carried out in the mixture containing 0.1 M pyrophosphate buffer pH8.5, 0.2 mM EDTA, 150 μM xanthine, and116 units/ml SOD (blue line), with additional 50 μM NaSCN plus 1 nM LPO (red line) or 10% bovine milk Fraction II (green line) at 25 °C. The reaction was started by addition of 20 nM XDH to the mixture. The increase in XO activity was monitored by the optical absorbance at 295 nm as O2-dependent urate formation. (C) Reaction mechanism for synergistic D→O. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)