FIGURE 1.
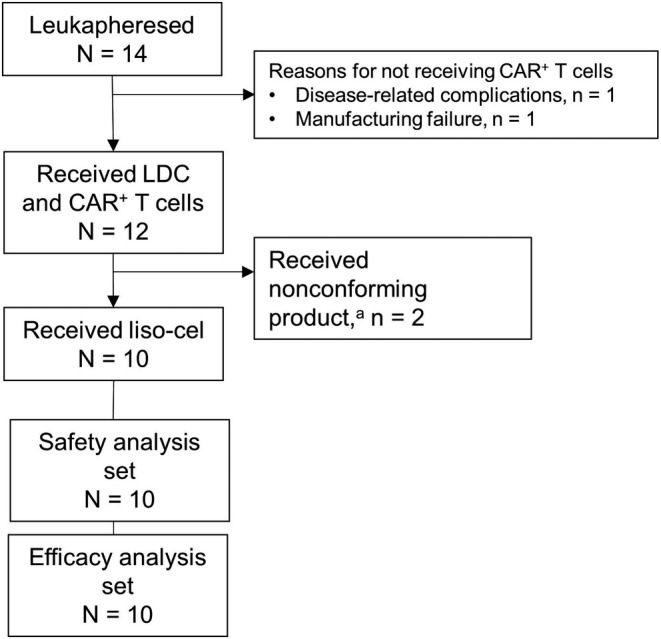
Patient flow. aLiso‐cel consists of equal target doses of CD8+ and CD4+ CAR+ T cells, each of which was required to meet quality specifications. Although CAR T cells could be manufactured for all but one patient, the product for two patients did not meet the specifications of liso‐cel (i.e., 1 of the CD8+ or CD4+ cell components did not meet one of the requirements to be considered liso‐cel). CAR, chimeric antigen receptor; LDC, lymphodepleting chemotherapy; liso‐cel, lisocabtagene maraleucel
