Introduction
Checkpoint inhibitors are monoclonal antibodies used in cancer therapy to stimulate the immune system’s antitumor activity by preventing T-cell inhibition through targets such as PD-1/PD-L1 and CTLA-4. The increased immune activity is not tumor-specific, and immune-related adverse events (irAEs) are common. Cutaneous reactions are some of the most frequent irAEs described, making up a broad spectrum of reactions, including acneiform, lichenoid, psoriasiform, and immunobullous eruptions,1,2 as well as rare checkpoint inhibitor-induced morphea or scleroderma-like syndrome (SLS).3,4 Some reported immunotherapy-induced SLS cases were severe and refractory to corticosteroid treatment, causing significant morbidity.4 This case describes the successful treatment of PD-1 inhibitor nivolumab-induced SLS with intravenous immunoglobulin (IVIG).
Case report
A 69-year-old woman with metastatic small-cell lung cancer on nivolumab for 2.5 years and a remote history of follicular lymphoma in remission initially presented to rheumatology with debilitating joint pain and swelling of the hands, wrists, feet, and ankles. She had a positive rheumatoid factor, which was negative the year prior. The patient was diagnosed with immunotherapy-induced rheumatoid arthritis, prompting the discontinuation of nivolumab. Sulfasalazine, methotrexate, hydroxychloroquine, and adalimumab were initiated with a 3-month course of prednisone. Two months into this regimen, the patient was referred to dermatology for skin tightening, dysphagia, and increasing fatigue. She denied symptoms of Raynaud's phenomenon. On examination, she had indurated and sclerotic plaques over her chest, arms, abdomen, and back with a limited range of motion (Fig 1). She had no nailfold capillary changes or sclerodactyly. Biopsy showed increased dermal collagen and entrapment of the eccrine glands consistent with morphea (Fig 2). Antinuclear antibody and extractable nuclear antigen panel were negative. Pulmonary function tests were consistent with restrictive lung disease. Mycophenolate mofetil and psoralen UV-A were added to the patient’s treatment regimen of sulfasalazine, methotrexate, hydroxychloroquine, adalimumab, and prednisone, but both were discontinued after 3 months because of intolerable nausea and vomiting. After about a year of treatment, persistent cytopenia resulted in the discontinuation of methotrexate. Her skin disease progressed with sclerotic plaques involving most of her upper body, with an associated decreased range of motion limiting her daily activities. She was started on IVIG and narrow-band UV-B (NBUVB). After 2 months, her cutaneous induration and tightening had improved, and the cobblestone texture of her lower extremities was less prominent. Ten months after starting IVIG and NBUVB, she had a dramatic improvement in her skin and range of motion (Fig 3). Because of COVID-19 exposure concerns, NBUVB therapy was tapered to once weekly and was eventually stopped. After stopping NBUVB, her skin disease remained well controlled for more than 15 months on monthly IVIG, adalimumab, and hydroxychloroquine. Palliative radiotherapy and cryoablation of a single adrenal tumor were the only treatments she received during this time for her lung cancer. The patient died suddenly from a myocardial infarction.
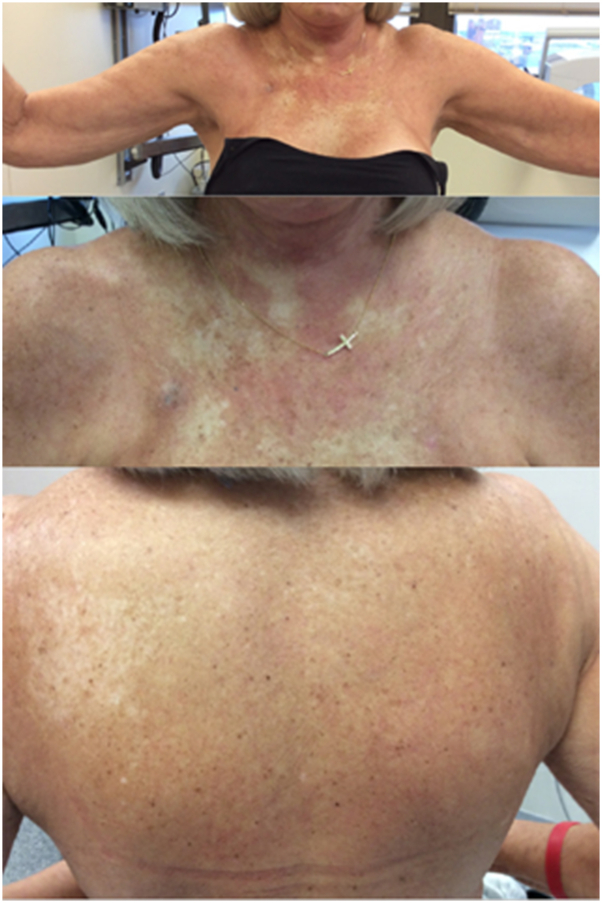
Fig 1.
Indurated, sclerotic plaques on the chest and back with markedly reduced range of motion.
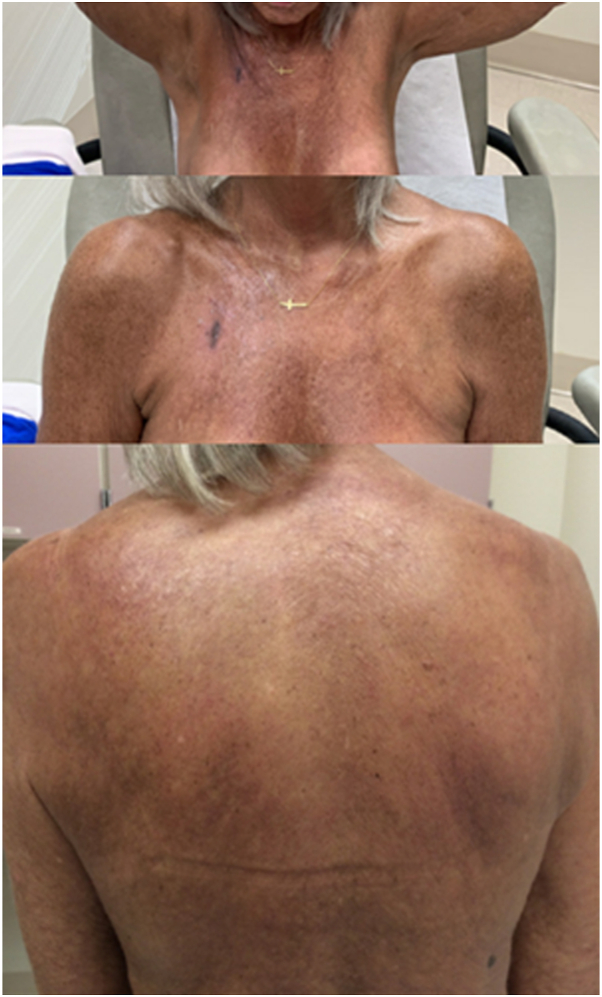
Fig 2.
Improved range of motion and reduction of induration and skin tightening after 10 months of IVIG therapy.
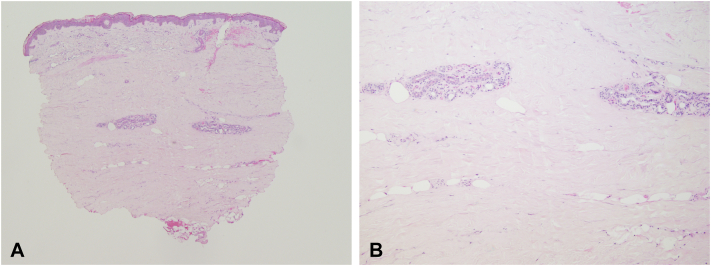
Fig. 3.
Square biopsy sign with increased dermal collagen and entrapment of the eccrine glands consistent with morphea.
Discussion
The patient presented here developed immunotherapy-induced SLS with overlapping features of generalized morphea and systemic sclerosis. Her cutaneous disease lacked some classic clinical features of scleroderma, including sclerodactyly, Raynaud’s phenomenon, and nailfold capillary changes. However, her dysphagia and restrictive lung disease coincided with the onset of her skin disease and were felt to be part of the same irAE. Given these overlapping features, we believe the best term for this irAE is nivolumab-induced SLS, a term used first in the description of another nivolumab-associated sclerotic skin disease that lacked some classic features of scleroderma.3 While a paraneoplastic SLS diagnosis was also taken into consideration, these patients typically do not improve without treatment of their underlying malignancy.5 Our patient improved on IVIG while treatment of her lung cancer was limited to palliative radiation before cryoablation of an individual metastasis. Therefore, a delayed nivolumab-induced SLS was thought more likely.
Current understanding of checkpoint inhibitor-induced fibrotic skin disease is limited but may involve upregulation of transforming growth factor β and the creation of a profibrotic state.2 Cutaneous irAEs may resemble other dermatologic conditions like morphea or scleroderma, but nuanced differences in the pathogenesis may impact patient presentation and response to therapy, shown by the contrasting degree of response to steroid therapy alone versus the requirement of additional therapies in sclerodermatous irAEs.2, 3, 4 In this case, steroid therapy was insufficient; the patient failed several additional therapies before responding to IVIG. As more checkpoint inhibitors are developed and used, the incidence of drug-induced fibrotic conditions will likely increase. Sclerodermatous irAEs have been reported after the use of dacarbazine,2 nivolumab,3,4 and pembrolizumab.2 However, it remains unclear whether the clinical presentation and treatment response of irAEs will be related to the specific offending drug. While most irAEs occur within a few months of therapy, delayed reactions are becoming increasingly recognized.6 Our patient developed SLS 2.5 years after starting nivolumab, adding to this growing list of delayed irAEs. For other cases of immune-related sclerotic skin disease see Table I.
Table I.
Other cases of immunotherapy-induced sclerotic skin disease
| Drug(s) | Target | Patient sex, age, and indication | irAE | Time from drug onset to iRAE | Treatment of SLS and result | SLS treatment response outcome |
|---|---|---|---|---|---|---|
| Pembrolizumab Ipilimumab Nivolumab |
PD-1, CTLA-4 | 61 M, Melanoma | Generalized morphea associated with eosinophilia4 | 10 mos (from combined immunotherapy onset) | Dexamethasone pulse dosing and clobetasone propionate | Improvement but not resolution |
| Nivolumab | PD-1 | 70 (s) M, Melanoma | SLS with no system symptoms5 | 54 wks | Prednisolone, 20 mg/d (0.3 mg/kg) | Resolution after 9-mo prednisone taper |
| Nivolumab | PD-1 | 61 M, Oligometastatic renal cell carcinoma | Scleroderma-like skin changes6 | 32 wks | Prednisone, mycophenolate mofetil | Improved; flared after prednisone taper requiring mycophenolate mofetil |
irAE, Immune-related adverse event; SLS, scleroderma-like syndrome.
Sclerotic conditions can have a profound impact on wellbeing, and no universally effective therapy exists. Systemic immunosuppressive therapies are often used, including methotrexate, mycophenolate mofetil, cyclophosphamide, prednisone, and rituximab, among others. IVIG offers a nonimmunosuppressive therapeutic option, which may be preferable during ongoing cancer treatment. The successful use of IVIG for systemic sclerosis was reported as early as 2000,7 and there are several reports of morphea demonstrating a positive response to IVIG therapy.8, 9, 10 Due to the relatively recent phenomenon of immunotherapy-induced SLS, it is unknown whether this condition will respond to the same therapies as systemic sclerosis and morphea. We present this case as an example of nivolumab-induced SLS responding to IVIG with marked improvement in disease burden and quality of life. It is worth noting that NBUVB may have initially contributed to improvement in our patient’s disease, however, IVIG likely had the greatest benefit as our patient did not experience a relapse when NBUVB was discontinued.
Though more research is needed to document the response rate and time until clinical improvement, we believe select patients may benefit from the addition or substitution of IVIG therapy for their SLS disease.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 2.Langan E.A., Budner K., Zillikens D., Terheyden P. Generalized morphoea in the setting of combined immune checkpoint inhibitor therapy for metastatic melanoma: A case report. Medicine (Baltimore) 2021;100(16) doi: 10.1097/MD.0000000000025513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho M., Nonomura Y., Kaku Y., et al. Scleroderma-like syndrome associated with nivolumab treatment in malignant melanoma. J Dermatol. 2019;46(1):e43–e44. doi: 10.1111/1346-8138.14492. [DOI] [PubMed] [Google Scholar]
- 4.Tjarks B.J., Kerkvliet A.M., Jassim A.D., Bleeker J.S. Scleroderma-like skin changes induced by checkpoint inhibitor therapy. J Cutan Pathol. 2018;45(8):615–618. doi: 10.1111/cup.13273. [DOI] [PubMed] [Google Scholar]
- 5.Jedlickova H., Durčanská V., Vašků V. Paraneoplastic scleroderma: are there any clues? Acta Dermatovenerol Croat. 2016;24(1):78–80. [PubMed] [Google Scholar]
- 6.Owen C.N., Bai X., Quah T., et al. 1138P Delayed immune-related adverse events (irAEs) on anti-PD1-based therapy. Ann Oncol. 2020;31:S761–S762. doi: 10.1016/j.annonc.2020.08.1261. [DOI] [Google Scholar]
- 7.Levy Y., Sherer Y., Langevitz P., et al. Skin score decrease in systemic sclerosis patients treated with intravenous immunoglobulin--a preliminary report. Clin Rheumatol. 2000;19(3):207–211. doi: 10.1007/s100670050158. [DOI] [PubMed] [Google Scholar]
- 8.Kucukoglu R., Yilmaz Z., Kutlay A. Treatment of recalcitrant generalized morphea with mycophenolate mofetil and intravenous immunoglobulin. Dermatol Ther. 2018;31(5) doi: 10.1111/dth.12674. [DOI] [PubMed] [Google Scholar]
- 9.Poelman C.L., Hummers L.K., Wigley F.M., Anderson C., Boin F., Shah A.A. Intravenous immunoglobulin may be an effective therapy for refractory, active diffuse cutaneous systemic sclerosis. J Rheumatol. 2015;42(2):236–242. doi: 10.3899/jrheum.140833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollina U., Looks A., Schneider R., Maak B. Disabling morphoea of childhood-beneficial effect of intravenous immunoglobulin therapy. Clin Exp Dermatol. 1998;23(6):292–293. doi: 10.1046/j.1365-2230.1998.00378.x. [DOI] [PubMed] [Google Scholar]