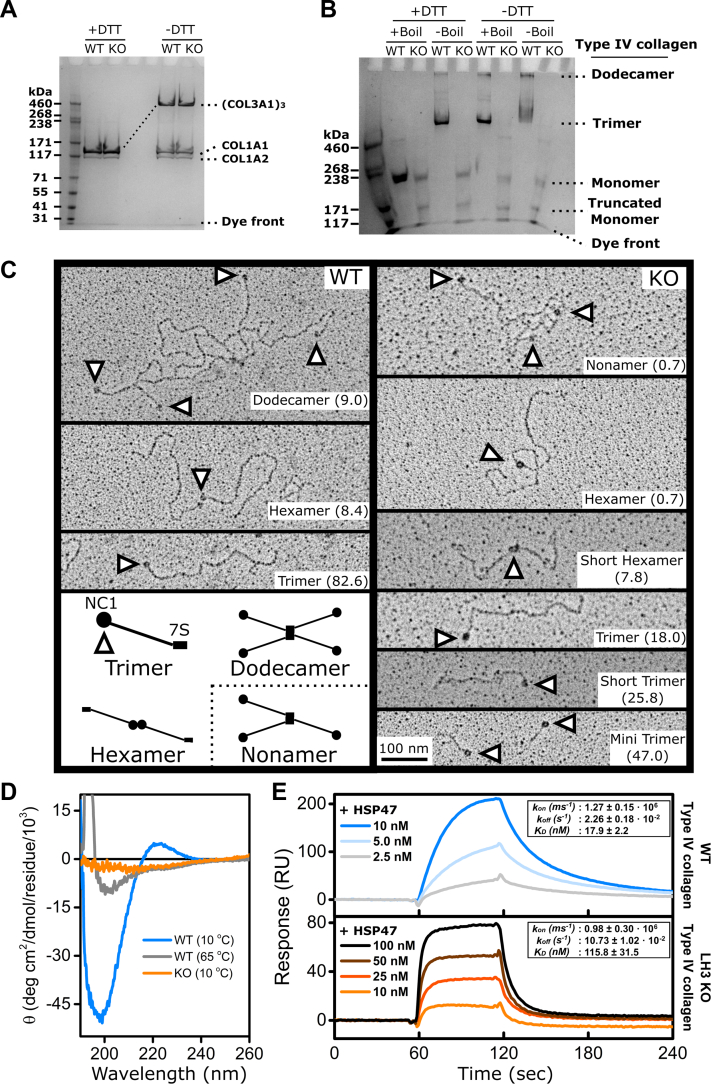
Figure 3.
LH3 deficiency impairs collagen α1α1α2(IV) oligomerization and triple helix formation.A, representative Coomassie blue–stained 3 to 8% Tris–acetate gel showing pepsin-treated collagens α1α1α2(I) and α1α1α1(III) purified from conditioned culture medium of WT and LH3 KO MEFs. B, representative Coomassie blue–stained 3.5% SDS-agarose gel showing collagen α1α1α2(IV) purified from conditioned culture medium of WT and LH3 KO PFHR9 cells. C, representative transmission electron rotary shadow microscopy images showing structural alterations of collagen α1α1α2(IV) purified from conditioned culture medium of WT and LH3 KO PFHR9 cells. Arrowheads point to globular NC1 domains. Diagram at the bottom left illustrates normal collagen α1α1α2(IV) network formation. The frequency of each molecular species is indicated in parentheses (%). D, CD spectra of purified collagen α1α1α2(IV) from conditioned culture medium of WT and LH3 KO PFHR9 cells under native (10 °C) and denaturing (65 °C) conditions. E, surface plasmon resonance analysis showing concentration-dependent binding kinetics between HSP47 and collagen α1α1α2(IV) purified from conditioned culture medium of WT and LH3 KO PFHR9 cells. Each curve represents the average of a minimum of three measurements. The binding constants and affinities are shown as mean ± SD in the inset. LH3, lysyl hydroxylase 3; MEF, mouse embryonic fibroblast.