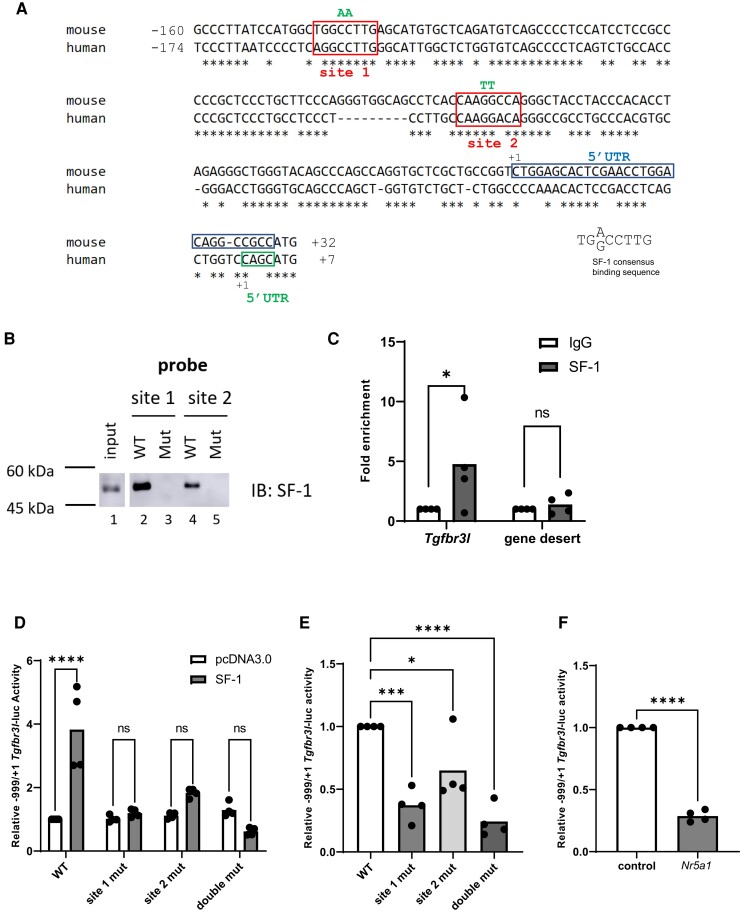
Figure 1.
SF-1 activates murine Tgfbr3l transcription via two cis-elements in the proximal promoter. (A) Alignment of the murine and human Tgfbr3l/TGFBR3L promoters. In both cases, +1 refers to the transcription start site. The most common murine 5′ untranslated region (5′ UTR) is boxed in blue and the human 5′ UTR is boxed in green. The conserved SF-1 binding sites are boxed in red and labeled as site 1 and site 2. Mutated base pairs (in B, C, and D) are indicated above in green. (B) DNAP using probes corresponding to the wild-type and mutant murine SF-1 cis-elements. Whole cell protein lysates from LβT2 cells (input) or proteins interacting with the probes were analyzed via immunoblot (IB) using an SF-1 antibody. (C) Chromatin immunoprecipitation for SF-1 of the indicated genomic regions in LβT2 cells (n = 4). (D) HEK293T cells were transfected with 225 ng/well of the indicated murine −999/+1 Tgfbr3l-luc reporters as well as 3.125 ng/well of either pcDNA3.0 (empty expression vector) or SF-1 expression vector. WT, wild-type; site 1 mut, mutated SF-1 site 1; site 2 mut, mutated SF-1 site 2; double mut, both SF-1 sites mutated. (E) LβT2 cells were transfected with 225 ng/well of the indicated promoter–reporters. (F) LβT2 cells were transfected with 225 ng/well of the −999/+1 Tgfbr3l-luc reporter and 10 nM of control or Nr5a1 siRNA. In D-F, lysates were collected and reporter activity measured by luciferase assay. Data represent the mean of three or more independent experiments performed in triplicate. Data were analyzed by two-way ANOVA followed by Holm–Sidak multiple comparisons test in (D), one-way ANOVA followed by Dunnett’s multiple comparisons test in (E), and two-tailed unpaired t test with Welch’s correction in (C and F). ns, not significant; *P < .05; ***P < .001; ****P < .0001.