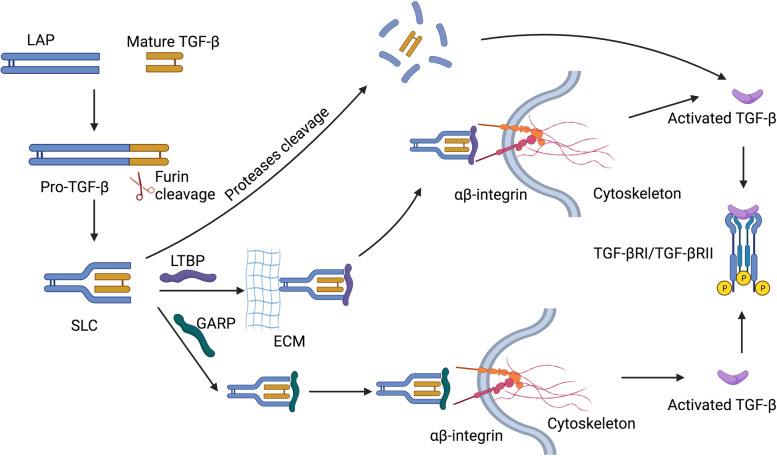
Fig. 1.
Schematic diagram and activation of latent TGF-β. The pro-TGF-β precursor consists of an N-terminal peptide with latency-associated peptide (LAP) and a mature C-terminal fragment. The pro-TGF-β precursor is cleaved by the convertase furin, and then the LAP dimer binds to mature TGF-β and forms the small latent complex (SLC). Proteases including plasmin, cathepsin and matrix metalloproteinase 9/14 (MMP9/14)) can cleave LAP and release active TGF-β in the extracellular matrix (ECM). SLC binds to latent TGF-β-binding protein (LTBP) with ECM proteins, including fibronectin and fibrillin, mediating the release of active TGF-β via interaction with αβ-integrin. TGF-β can also be activated through SLC anchoring to glycoprotein A repetition predominant protein (GARP)