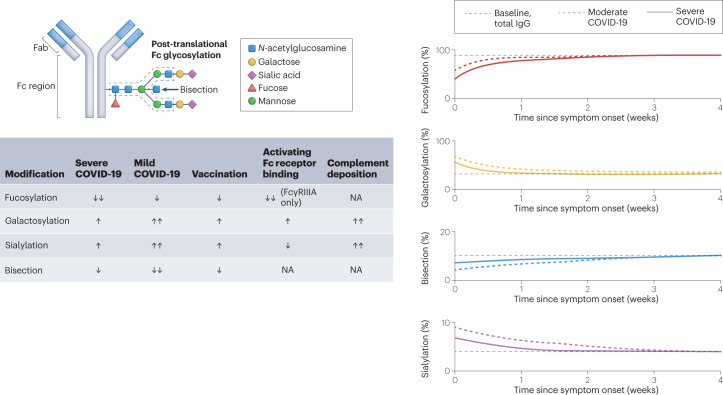
Fig. 2. Antibody glycoforms are shaped by infection and vaccination, and modulate Fc-dependent effector functions.
The crystallizable fragment (Fc) regions of antibodies are post-translationally modified with patterns of glycosylation that shape their function. The relative levels of fucosylation, galactosylation, sialylation and bisection of antibody Fc regions have all been reported to affect the outcome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Severe coronavirus disease 2019 (COVID-19) is associated with transient decreases in fucosylated antibodies that return to baseline levels of fucosylation 4–6 weeks after infection. Antibody afucosylation results in more potent Fc receptor (FcR) activation, specifically of FcγRIIIA. By contrast, galactosylation levels are increased in response to infection and vaccination. Antibody galactosylation enhances FcR activation, albeit to a lower level than does afucosylation, but is associated with more potent complement deposition. Antibody sialylation also increases upon vaccination or infection. Sialylation slightly reduces binding to activating FcRs, but enhances complement deposition. By contrast, levels of bisection slightly decrease with infection and vaccination, although the biological relevance of this is unclear as neither FcR binding nor complement deposition seem to be affected. The graphs in the right-hand panel depict the percentage of total or anti-spike IgG with specific glycosylation patterns in patients at baseline, with moderate COVID-19 (hospitalized, non-intensive care) or with severe COVID-19 (hospitalized, intensive care) following weeks after symptom onset. Fab, fragment antigen-binding. NA, no effect observed in response to changes in modification level.