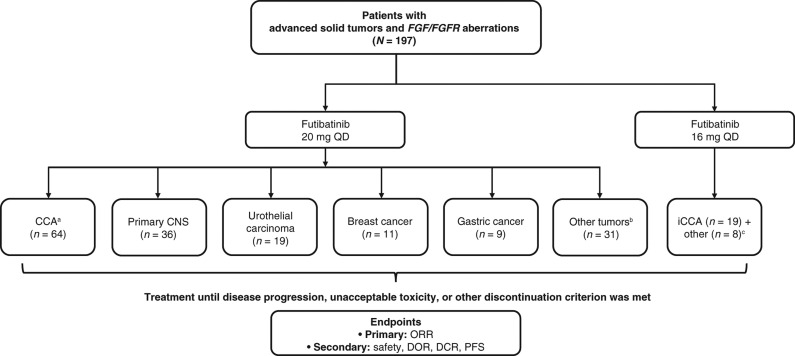
Figure 1.
Phase I expansion study design. aIntrahepatic (n = 61) and extrahepatic (n = 3) CCA. bSarcoma (n = 6); colorectal cancer (n = 5); endometrial cancer (n = 3); esophageal cancer (n = 3); gallbladder cancer (n = 3); head and neck cancer (n = 2); adrenal cortical cancer, lung cancer, mesothelioma, ovarian cancer, pancreatic cancer, and thyroid cancer (n = 1 each); and primary unknown (n = 3). cBreast cancer, gallbladder cancer, primary CNS cancer, sarcoma, urothelial cancer, and thyroid cancer (n = 1 each), and primary unknown (n = 2). iCCA, intrahepatic CCA; QD, once daily.