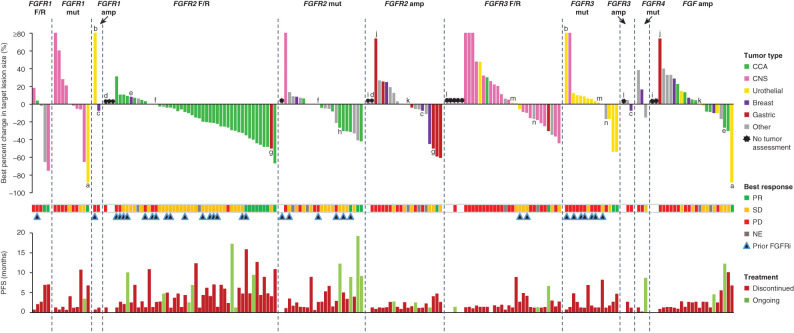
Figure 3.
Individual response and treatment outcome by FGFR aberration in patients who received futibatinib 20 mg once daily. The figure shows individual treatment outcomes organized by FGFR aberration type, color coded for tumor type in patients who received futibatinib 20 mg once daily. RECIST v1.1 criteria were used for tumor response assessment for all tumor types except for CNS tumors, for which RANO criteria were used. Several patients (n = 14) had more than one type of FGF/FGFR aberration and are represented in each relevant FGFR aberration category. These patients are indicated with the letters a–n, with each letter representing an individual patient.