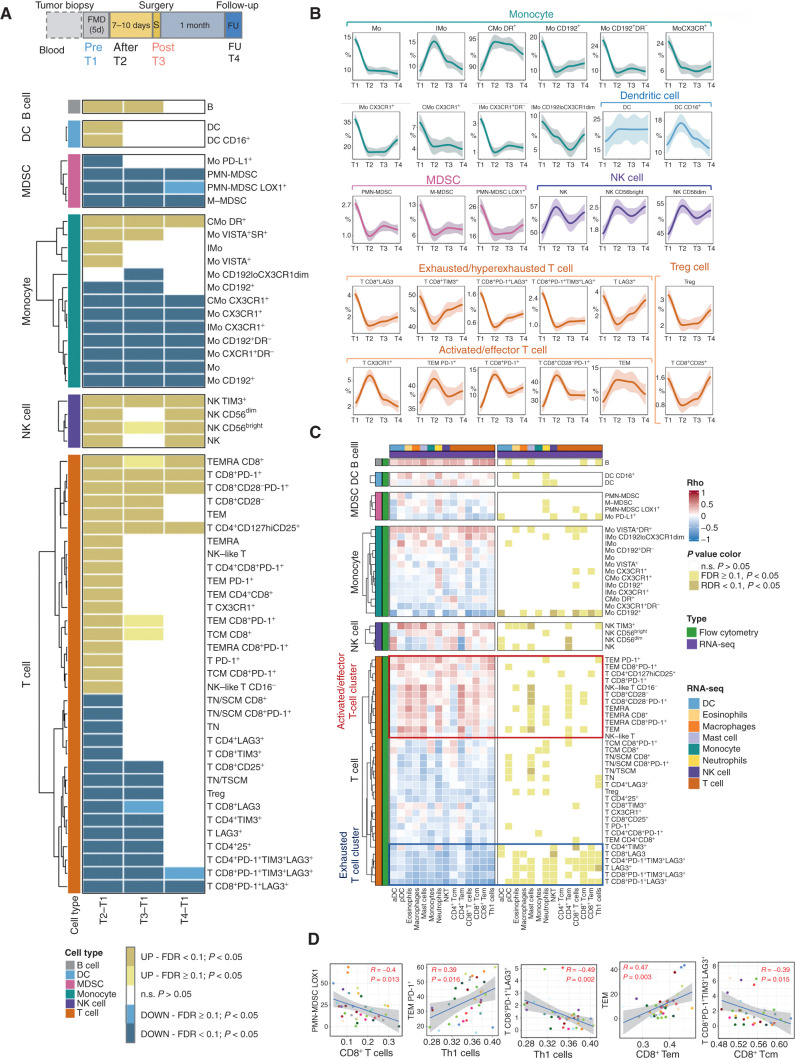
Figure 5.
Dynamic effects of FMD on systemic immunity and correlation with transcriptional immune modulation at tumor site. High-dimensional flow cytometry was performed in PBMCs from 19 of 22 patients enrolled in the DigesT trial selected for tumor RNA-seq analysis. A, Samples for high-dimensional flow cytometry were collected before the FMD (T1) and at different time points after the FMD (T2, T3, T4). We assessed the expression of multiple lymphoid and myeloid markers to define a total of 120 cell subsets (see Supplementary Fig. S11–S13). The nonparametric paired Wilcoxon test was used to compare the diverse immune cell frequencies at T2 versus T1, T3 versus T1, and T4 versus T1; 57 PBMC subpopulations undergoing modifications passing the significance cutoff at least at one time point (P < 0.05, Benjamini-Hochberg FDR < 0.1) are represented in the heat map. B, Loess regression curves of representative immune cell subsets are shown to illustrate modulation across time. See Supplementary Fig. S14 for the representation of the remaining PBMC Loess regression curves related to the significantly modulated PBMC subsets. C, Heat map representing Spearman correlations between significantly modulated blood PBMC subpopulations (n = 57) and intratumor leucocyte subsets (n = 14) at T3 versus T1 in 19 patients for whom both PBMC flow cytometry and tumor RNA-seq data were available. D, Representative correlations between individual patient values of the indicated PBMC and intratumor leukocyte subsets (P < 0.05, Benjamini–Hochberg FDR < 0.1) at T1 and T3.