FIGURE 1.
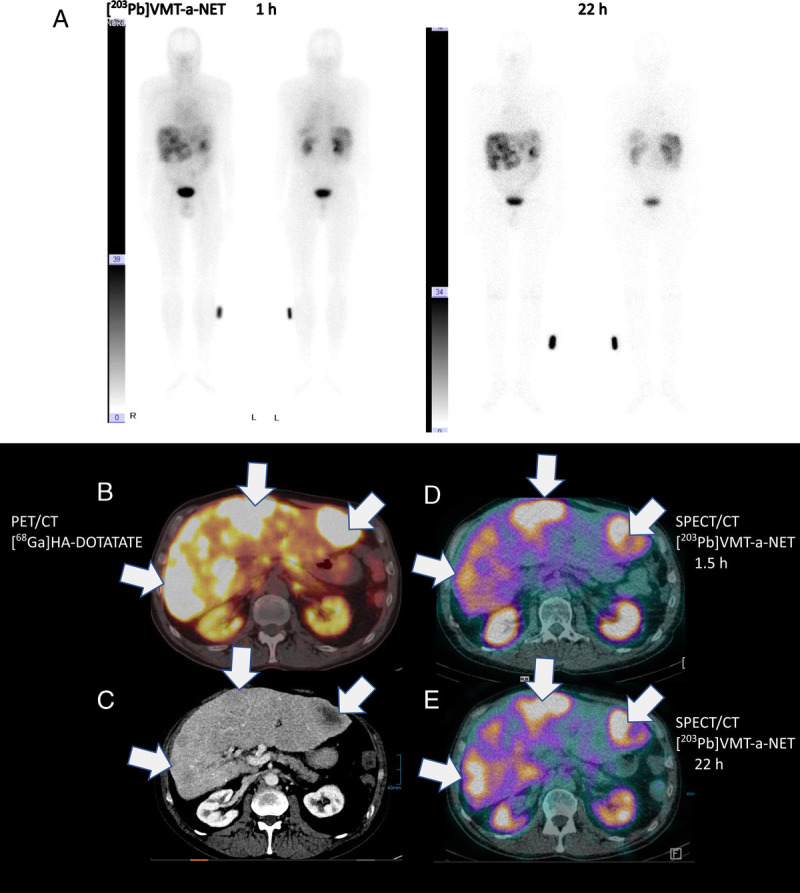
Tyr3-octreotide (TOC) variant VMT-α-NET is a novel and ideal ligand for 203Pb/212Pb that shows lower renal excretion, higher neuroendocrine tumor uptake, and high chelation properties for retention of daughter α-particle emitting radionuclide 212Bi. In an end-stage midgut neuroendocrine tumor patient with carcinoid heart disease, right ventricular dysfunction, mildly reduced renal function, and refractory to 6 cycles of 177Lu-HA-DOTATATE therapy, planar, and 22 hours 203Pb-VMT-α-NET SPECT/CT (low dose) images were acquired after injection of 224 MBq of 203Pb-VMT-α-NET to assess the feasibility of performing 212Pb-VMT-α-NET therapy. The patient tolerated the injection without any significant alteration in the vital parameters. There was rapid renal clearance of the tracer within the first hour itself as was evident from tracer excretion in the urinary bladder and in the renal pelvicalyceal system. Because of known right ventricular dysfunction, there was evidence of blood pool activity in the heart, which however decreased significantly after 21 hours. A comparison of the 1.5 and 22 hours SPECT/CT images with 68Ga-HA-DOTATATE PET/CT images (contrast-enhanced diagnostic CT) showed high uptake of 203Pb-VMT-α-NET in liver metastases matching with the results of the PET/CT. A, Anterior and posterior planar images acquired at 1 and 22 hours. B, Fused 68Ga-HA-DOTATATE PET/CT transverse slice. C, Transverse contrast-enhanced CT image. D and E, SPECT/CT (low-dose) fused transverse slice. Arrows are showing the liver metastases.1–8
