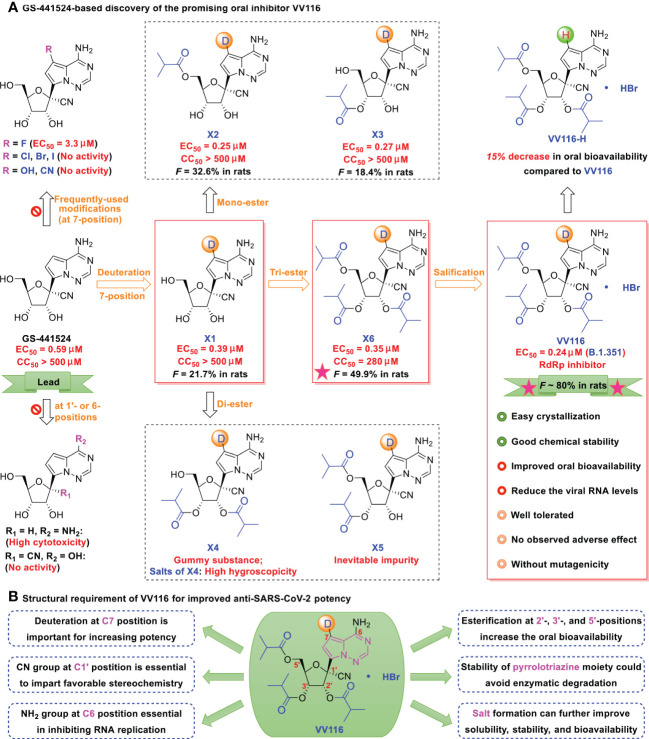
Figure 3.
(A) GS-441524-based discovery of the promising oral inhibitor VV116. GS-441524 presents a promising drug candidate for further drug development. Several previous failed attempts are summarized: (i) introduction of frequently-used halogen (F, Cl, Br, I), hydroxyl, or cyano groups at the C7-position, resulting in less potent or no anti-SARS-CoV-2 activity, (ii) removal of the cyano group at C1′-position, results in high cytotoxicity, (iii) changing the 6-amino to a hydroxyl, methylation of the 2’-α-hydroxyl, or changing the 2’-α-hydroxyl to fluorine, results in loss of anti-SARS-CoV-2 activity. Promising attempts: deuteration at the C7 position results in strong antiviral activity; induces mono-, di- and tri-esters at the 2′-, 3′-, and 5′-positions, and improves oral bioavailability (tri-isobutyrate ester X6 [F ~ 50% in rats] was superior to the others in rats]); and induces hydrobromide in X6, yielding the promising oral candidate VV116 (white solid, with improved oral bioavailability, good chemical stability; no observed adverse effect, and no mutagenicity). (B) Structural requirement of VV116 for improved anti-SARS-CoV-2 potency.