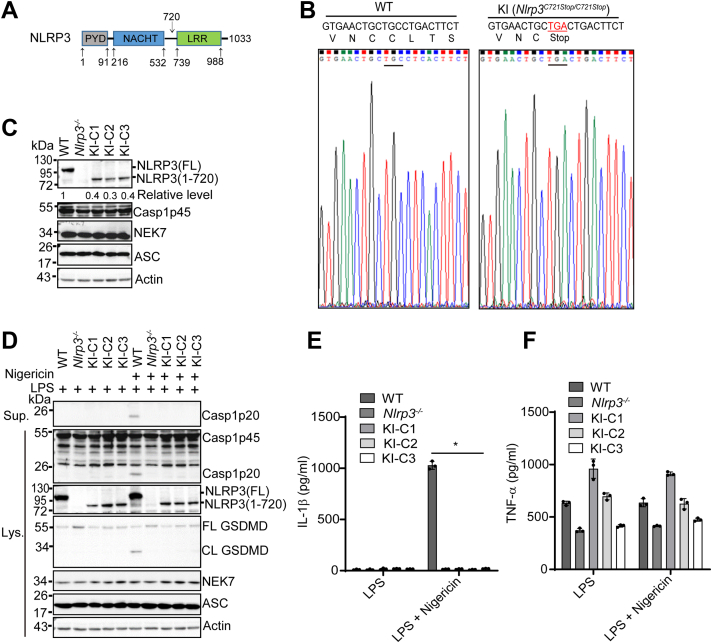
Figure 1.
The LRR domain is required for nigericin-induced endogenous NLRP3 inflammasome activation in macrophages.A, schematic presentation for domains of mouse NLRP3 (UniProtKB: Q8R4B8). The bottom numbers show the positions of amino acid residues at the start and end of each domain. The top number (720) shows the position of the last amino acid residue for an NLRP3 LRR deletion mutant [NLRP3 (1–720)]. B, sequencing verification for the replacement of a cysteine codon (TGC) by a stop codon (TGA) in mouse Nlrp3 gene. C, immunoblot analysis of NLRP3 inflammasome components in wildtype (WT), Nlrp3−/−, and knock-in macrophages (three individual clones: KI-C1, KI-C2, KI-C3). Actin was used as a control. Representative blots (n = 2). The band density of NLRP3 was measured by ImageJ and normalized to the full-length NLRP3. D, macrophages were stimulated with LPS (4 h) alone or plus 5 μM nigericin (1 h). Cell lysates and supernatants were immunoblotted with indicated antibodies. Representative blots (n = 3). Measurement of IL-1β (E) and TNF-α (F) in the supernatants from stimulated macrophages by ELISA. Representative data(n = 3). ∗p < 0.05 (one-way ANOVA). Data are the mean ± SD of triplicate wells. CL, cleaved; FL, full-length; LPS, lipopolysaccharide; LRR, leucine-rich repeat.