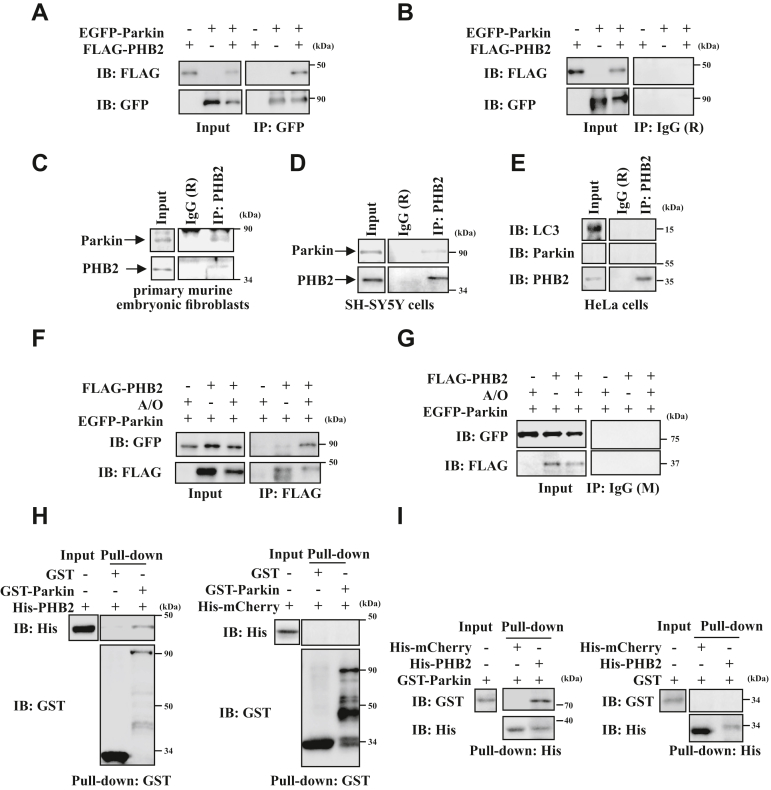
Figure 1.
Parkin directly interacts with PHB2, and the interaction is enhanced during mitophagy.A and B, HEK293T cells expressing EGFP vector or EGFP-Parkin with FLAG vector or FLAG-PHB2 were treated with 5 μg/ml A/O (Antimycin A and Oligomycin A) for 4 h. Cell lysates were subjected to immunoprecipitation analysis using anti-GFP antibody (A) or anti-IgG (R) antibody (B). Co-immunoprecipitated FLAG-PHB2 was detected using anti-FLAG antibody, n = 3. C–E, primary murine embryonic fibroblasts cells (C), SH-SY5Y cells (infected with lentivirus carrying mCherry-Parkin) (D) or HeLa cells (E) were treated with 5 μg/ml A/O for 4 h, and then cell lysates were subjected to immunoprecipitation analysis using anti-PHB2 antibody. Co-immunoprecipitated proteins were detected using anti-Parkin and anti-LC3 antibodies, n = 3. F–G, HEK293T cells expressing FLAG vector or FLAG-PHB2 with EGFP-Parkin were treated with DMSO or 5 μg/ml A/O for 4 h. Cell lysates were subjected to immunoprecipitation analysis using anti-FLAG antibody (F) or anti-IgG (M) antibody (G), and co-immunoprecipitated EGFP-Parkin was detected with anti-GFP antibody, n = 3. H–I, after purification by GST (H) or His (I) binding resin, GST-tagged (H) or His-tagged (I) proteins were separately mixed with His-PHB2 (H, left region), His-mCherry (H, right region), GST-Parkin (I, left region), or GST (I, right region). 10% input was loaded as control. The bound proteins were analyzed by immunoblot analysis with anti-GST or anti-His antibodies, n = 3. EGFP, enhanced green fluorescent protein; PHB2, prohibitin 2.