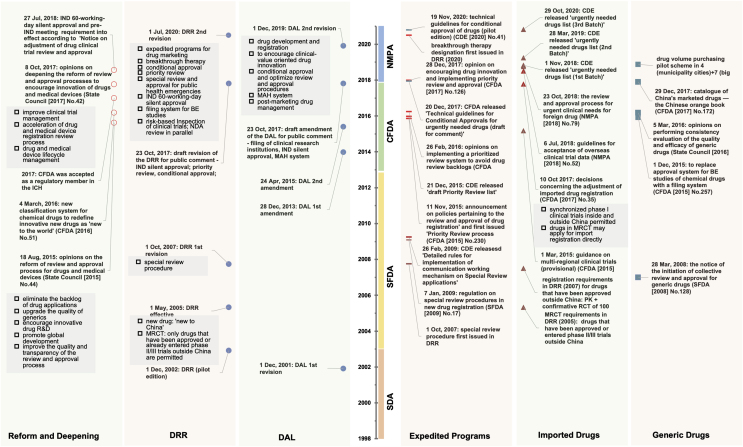
Figure 1.
Iteration of China’s key pharmaceutical laws and policies, 1998–2021. Major policies and announcements were grouped according to their main objectives and effects: reform and deepening, drug registration regulation (DRR), drug administration law (DAL), expedited programs, imported drug policies, and generic drug policies. SDA, State Drug Administration; SFDA, State Food and Drug Administration; CFDA, China Food and Drug Administration; NMPA, National Medical Products Administration; CDE, Center for Drug Evaluation; ICH, International Council for Harmonization; IND, investigational new drug; NDA, new drug application; MRCT, multi-regional clinical trials; MAH, Marketing Authorization Holder.