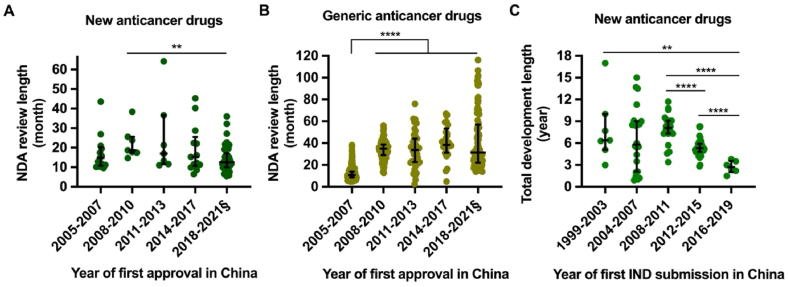
Figure 2.
Trend of NDA review length and total development length of new anticancer drugs approved in China, 2005 to 2021. The new drug application (NDA) review length is shown by the time between NDA submission and approval among new anticancer drugs (A) and generic drugs (B). Drugs were grouped into quintiles of the first approval year in China spanning from January 2005 to May 2021. Only four generic drugs (no new anticancer drugs) were approved in 2016; therefore, drugs approved between 2014 and 2017 were presented as a group. The total development length of new anticancer drugs (C) is shown by the time between the submission of investigational new drug (IND) application to first approval in China. Drugs are grouped according to the year of first IND submission in China. Horizontal lines indicate medians, and error bars depict interquartile ranges. §Data until 31 May 2021. ∗∗P < 0.01, ∗∗∗∗P < 0.0001.