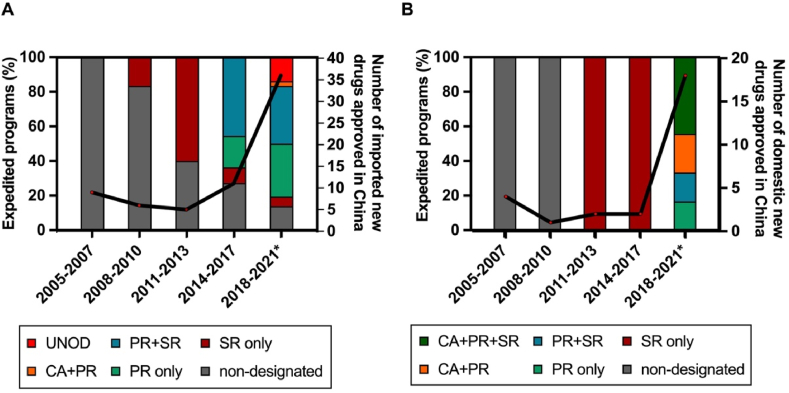
Figure 3.
Uptake of expedited programs (EP). The proportion of EP designations and overall number of imported (A) and domestic (B) new anticancer drugs are shown in three-year intervals from January 2005 to May 2021. SR, Special Review designation, effective between October 2007 and June 2020; PR, Priority Review, effective from November 2015; CA, Conditional Approval, effective since December 2017; UNOD, Urgently Needed Overseas Drugs, three batches of UNOD released since November 2018. ∗Data until 31 May 2021.