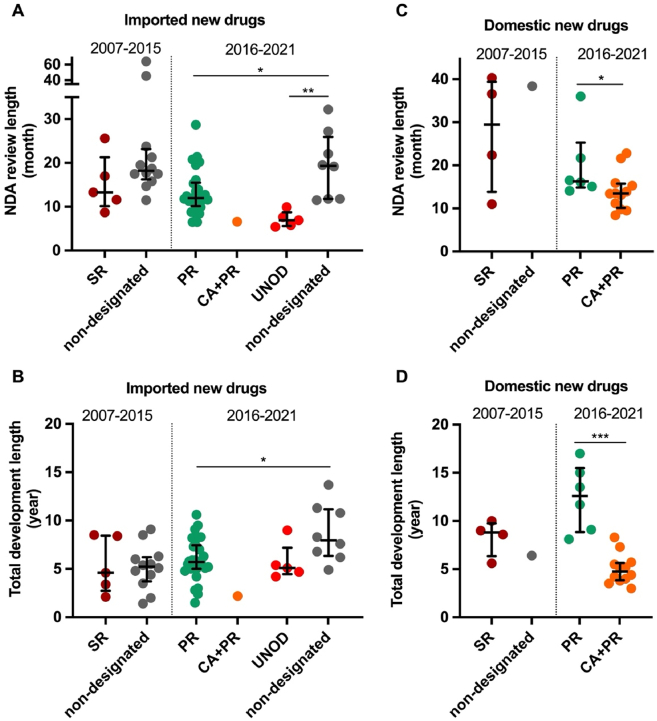
Figure 4.
NDA review length and total development length of approved new anticancer drugs in China by expedited programs, 2007 to May 2021. The new drug application (NDA) review length (month) is shown by the time between NDA submission and approval for imported (A) and domestic new drugs (C), and the total development length (year) is shown by the time between the submission of investigational new drug (IND) application to first approval in China for imported (B) and domestic (D) new drugs, respectively. Dark red circles denote anticancer drugs granted Special Review (SR) designation from October 2007 to December 2015. Green, bright red, and orange circles denote anticancer drugs with Priority Review (PR), Urgently Needed Overseas Drugs (UNOD), and PR plus conditional approval (CA) designations, respectively, since Dec 2015. Comparators (non-SR or non-PR/UNOD) are approval year-matched anticancer drugs without expedited programs designation. Horizontal lines indicate medians, and error bars depict interquartile ranges. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.