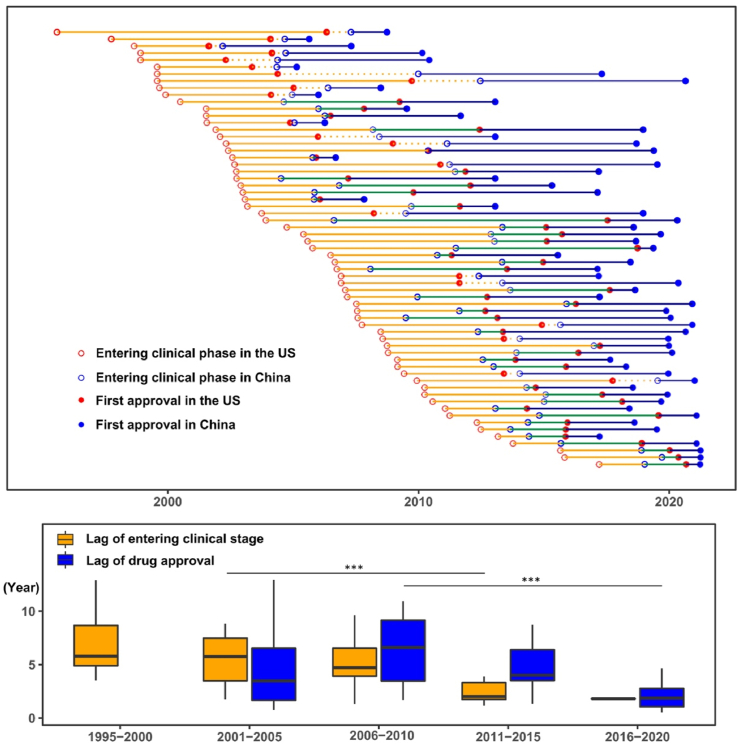
Figure 5.
Clinical development and first approval of imported new anticancer drugs in the US and China. Clinical development and approval dates are shown by clinical development and registration strategies and development timelines (five-year interval from 1995 to 2021). Each line represents one drug approved in China between Jan 2005 to May 2021 that was approved in the US after 2000. The red and blue hollow circles represent the start date of first clinical trial in the US and dates of investigational new drug (IND) submission in China. Red and blue solid circles are dates of first approval in the US and China, respectively. Orange line segments (from red hollow circle to blue hollow circle) indicate the time difference of entering clinical stage between the US and China, which is plotted as orange boxes on the bottom by five-year intervals. Similarly, blue line segments (from red solid circle to blue solid circle) indicate the lag of drug approval between the US and China, which is further plotted as blue boxes. ∗∗∗P < 0.001.