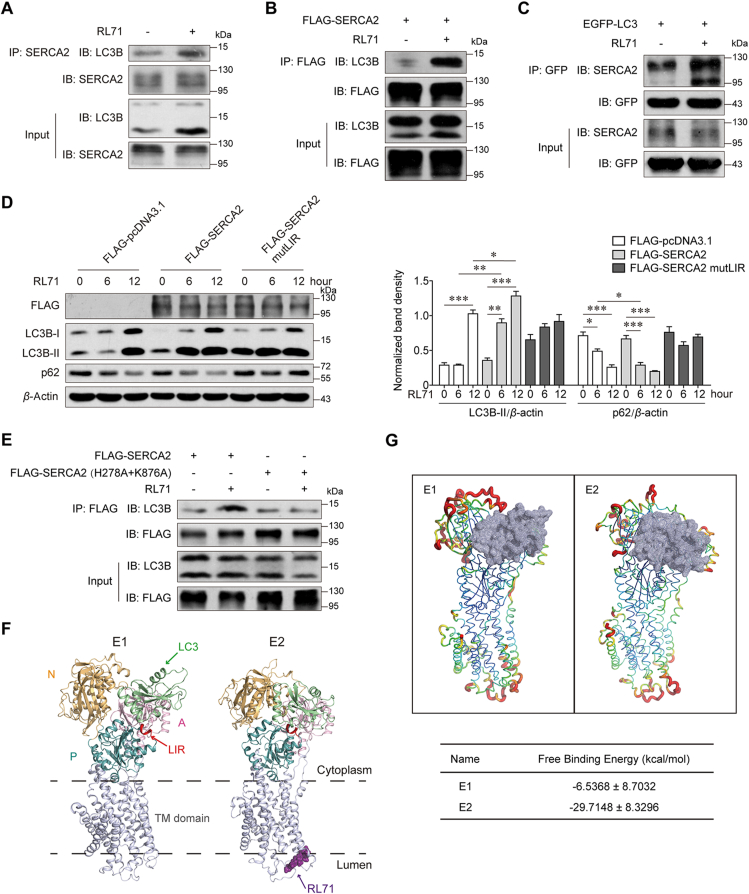
Figure 6.
The small-molecule RL71 promotes SERCA2–LC3B interaction. (A) Co-IP of SERCA2 with endogenous LC3B in SUM1315 cells 6 h after the treatment of 2 μmol/L RL71. (B) Co-IP of FLAG-SERCA2 with endogenous LC3B and (C) Co-IP of EGFP–LC3 with endogenous SERCA2 in the transfected SUM1315 cells 6 h after the treatment of 2 μmol/L RL71. (D) Western blot analysis of p62 and LC3B in SUM1315 cells, which were transfected with the indicated plasmids and then treated with 2 μmol/L RL71 for 0–12 h. (E) Co-IP of FLAG-SERCA2 or its mutant with endogenous LC3B in SUM1315 cells, which were transfected with the indicated plasmids and then treated with 2 μmol/L RL71 for 6 h. (F) Ribbon representation of SERCA2 in the LC3-bound E1 form and that in E2 form bound with both LC3 and RL71. The cytoplasmic part of SERCA2 consists of N domain (yellow), P domain (blue) and A domain (purple)30. LIR (red) in P domain interacts with LC3 (green). RL71 (dark purple) binds to SERCA2 at the cleft on the luminal side of the ER14. TM, transmembrane. (G) Cartoon representation of SERCA2 in E1 or E2 form bound with LC3 (gray). Red indicates high flexibility and green indicates low flexibility. Data are shown as the mean ± SD of at least three independent experiments. ∗P < 0.05, ∗∗P < 0.005, ∗∗∗P < 0.001.