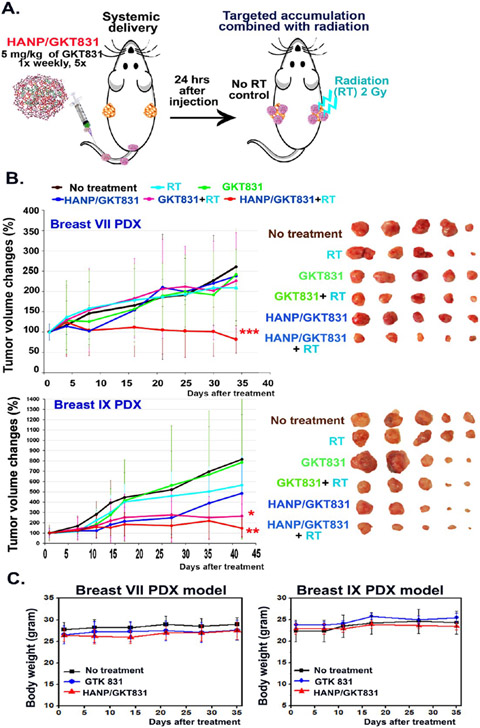
Figure 4.
Determination of therapeutic efficacy of HANP/GKT831 without or with in combination with radiation therapy in orthotopic breast PDX tumor models. (A) Treatment protocol. Nude mice bearing two orthotopic breast PDX tumors on the right and left mammary fat pads received 5 mg/kg of GKT831 equiv dose of HANP/GKT831 via the tail vein injections once per week for five weeks. 2 Gy of radiation was given to the right-side tumor 24 h after each HANP/GKT831 injection, while the left-side tumors were shielded. (B) (left) Relative tumor growth curves following different treatments in Breast VII and Breast IX PDX tumor models in nude mice. (right) PDX-tumor images of treatment groups. Student’s t-test: *p < 0.05, **p < 0.01, ***p < 0.005. Breast VII PDX: No-treatment control vs HANP/GKT831+RT: p = 0.002; HANP/GKT831+RT vs RT: p = 0.004 or vs GKT831+RT: p = 0.047. Breast IX PDX: No treatment control vs RT: p = 0.39; HANP/GKT831+RT vs no treatment: p = 0.0085 or vs RT: p = 0.015. No-treatment control vs HANP/GKT831: p = 0.07, or vs GKT831+RT: p = 0.03. n = 5 mice/group. (C) Determination of systemic toxicity by monitoring body weights.