Abstract
Background
Guideline-directed medical therapy (GDMT) improves clinical outcomes in patients with heart failure with reduced ejection fraction (HFrEF). Despite its proven efficacy, GDMT is underutilized in clinical practice. The current study examines GDMT utilization after incident hospitalization for HF to promote medication initiation, and titration to target dosing within a reasonable time period.
Methods
This observational study identified 66,372 patients with HFrEF who were aged ≥ 65 years and had an incident HF hospitalization, using administrative health data (2013-2018). GDMT (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor-neprilysin inhibitors, β-blockers (BB), and mineralocorticoid receptor antagonists ) received within the 6 months after hospitalization was evaluated by monitoring therapy combinations, optimal dosing (proportion receiving ≥ 50% of the target dose for these inhibitors and blockers, and any dose of MRA), and maximal and last dose assessed, and by use of a GDMT intensity score.
Results
Among patients with HFrEF, 4768 (7.2%) were on no therapy, 17,184 (25.9%), were on monotherapy, 30,912 (46.6%) were on dual therapy, and 13,508 (20.4%) were on triple therapy. Only 8747 (13.2%) and 5484 (8.3%) achieved optimal GDMT based on the maximum dose and the last dispensed dose, respectively, within 6 months postdischarge. Finally, 38,869 (58.6%) achieved < 50% of the maximum intensity score, 23,006 (34.7%) achieved between 50% and 74% of the maximum intensity score, and 4497 (6.8%) achieved a score that was ≥ 75% of the maximum intensity score.
Conclusions
Current pharmacologic management for patients with HFrEF does not align with the Canadian guidelines. Given this gap in care, innovative strategies to optimize care in patients with HFrEF are needed.
Résumé
Introduction
Les traitements médicamenteux préconisés dans les lignes directrices (TMPLD) permettent d’améliorer les résultats cliniques des patients atteints d’insuffisance cardiaque à fraction d’éjection réduite (ICFER). En dépit des preuves de leur efficacité, les TMPLD sont sous-utilisés dans la pratique clinique. La présente étude porte sur l’utilisation des TMPLD après une hospitalisation incidente en raison d’une IC afin de favoriser l’amorce de la médication, et l’ajustement de la posologie en vue d’atteindre la dose cible dans un délai raisonnable.
Méthodes
Cette étude observationnelle portait sur 66 372 patients atteints d’ICFER qui avaient ≥ 65 ans et une hospitalisation incidente en raison d’une IC, et reposait sur les données administratives sur la santé (2013-2018). Nous avons évalué les TMPLD (inhibiteurs de l’enzyme de conversion de l’angiotensine, bloqueurs des récepteurs de l’angiotensine, β-bloquants [BB] et antagonistes des récepteurs des minéralocorticoïdes [ARM]) reçus dans les six mois après l’hospitalisation par la surveillance des combinaisons de traitement, la posologie optimale (proportion recevant ≥ 50 % de la dose cible pour ces inhibiteurs et ces bloquants, et toute dose d’ARM), la dose maximale et la dernière dose évaluées, et par l’utilisation d’un score d’intensité des TMPLD.
Résultats
Parmi les patients atteints d’ICFER, 4 768 (7,2 %) n’avaient reçu aucun traitement, 17 184 (25,9 %), avaient reçu une monothérapie, 30 912 (46,6 %) avaient reçu une bithérapie et 13 508 (20,4 %) avaient reçu une trithérapie. Seuls 8 747 (13,2 %) et 5 484 (8,3 %) avaient obtenu les TMPLD optimaux en fonction de la dose maximale et de la dernière dose administrée, et ce, respectivement, dans les six mois après la sortie de l’hôpital. Enfin, 38 869 (58,6 %) avaient obtenu < 50 % du score d’intensité maximale, 23 006 (34,7 %) avaient obtenu entre 50 % et 74 % du score d’intensité maximale, et 4 497 (6,8 %) avaient obtenu un score qui était ≥ 75 % du score d’intensité maximale.
Conclusions
La prise en charge pharmacologique actuelle des patients atteints d’ICFER va à l’encontre des lignes directrices canadiennes. Compte tenu de cette lacune dans les soins, des stratégies novatrices pour optimiser les soins aux patients atteints d’ICFER sont nécessaires.
With a yearly incidence of 50,000 cases affecting approximately 600,000 Canadians, heart failure (HF) is a major healthcare problem.1 Guideline-directed medical therapy (GDMT) with angiotensin-converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), angiotensin receptor-neprilysin inhibitors (ARNIs), β-blockers (BBs), and mineralocorticoid receptor antagonists (MRAs) have shown mortality and morbidity benefit in cases of heart failure with reduced ejection fraction (HFrEF) in several landmark trials.2, 3, 4, 5, 6, 7 Unfortunately, observational studies of patients with HFrEF have shown suboptimal initiation of HF-related pharmacotherapy postdiagnosis.1,8, 9, 10
Several methods to evaluate GDMT have been proposed, including medication intensity scores,11 opportunistic assessments, and simple counting of the number of medications in a class. Although summative methods (eg, simple addition of a class as on/off) have advantages, they fail to account for dosing, which plays a major role in assessment of the quality of care. The current study utilizes dosing data and intensity scores to examine successful dose titration of GDMT throughout the study period.
The aim of the current study was to examine the GDMT utilization in Canada in patients with HFrEF and a recent hospitalization, as defined by the Canadian Cardiovascular Society (CCS) guidelines for HF management.11,12 We explored trends of GDMT use over time and the various combinations of medical therapy used; additionally, we explored GDMT dosing using a GDMT intensity score.
Methods
Study design and data source
We conducted a population-based retrospective cohort study using the Discharge Abstract Database and the National Prescription Drug Utilization Information System (NPDUIS) datasets from the Canadian Institute for Health Information. The Discharge Abstract database contains data on admission dates, discharge dates, discharge disposition, primary and secondary diagnoses, procedures, and demographic information for all patients admitted to an acute care hospital in Canada, except for the province of Quebec. Diagnoses are coded using the International Classification of Diseases, version 10 (ICD-10), and procedures are coded using the Canadian Classification of Health Interventions. The National Prescription Drug Utilization Information System database contains drug dispensing data for adult Canadians covered by their provincial plan, except for those from Quebec, Nova Scotia, and the territories (Northwest Territories, Nunavut, and Yukon). Coverage varies across provinces, but all provinces cover those aged 65 years and older. The database contains drug dispensing dates, drug description including drug dose, anatomic therapeutic chemical drug classification, drug supply, and number of tablets/capsules dispensed. Data were linked longitudinally within and across datasets using a unique and anonymous patient identification number.
This study was approved by the University of Alberta Research Ethics Board (Pro00040008).
Patient selection
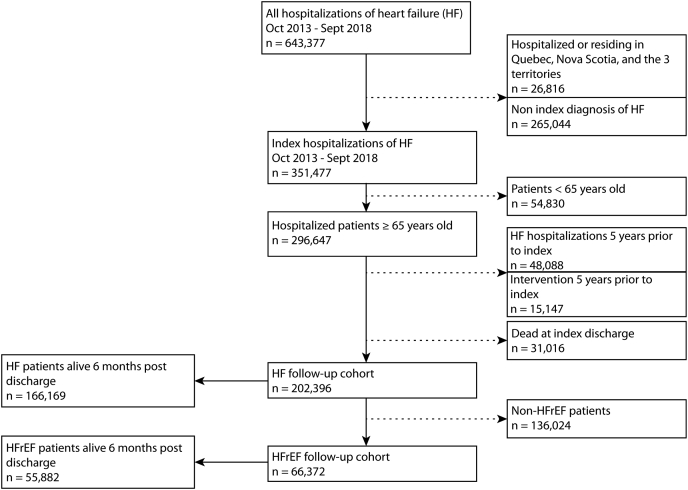
Patients aged ≥ 65 years with HF-related hospital admissions between October 1, 2013 and September 30, 2018 were identified using ICD-10 code I50.x as a primary or secondary diagnosis and were followed-up for 6 months postdischarge. The study period and age criteria were selected to allow data availability on drug prescriptions. Specifically, only patients aged ≥ 65 years have universal drug coverage in Canada, thereby removing variables such as affordability of drugs, which many be a factor for those aged < 65 years. Patients who were hospitalized, or who were residing in the provinces of Quebec, Nova Scotia, and the territories, were excluded, as medication claims were not available for them. In patients with multiple HF admissions during the study period, the first admission was considered the index admission. To ensure that prevalent cases of HF were excluded, patients who had a diagnosis of HF, any record of cardiac resynchronization therapy, an implantable cardioverter defibrillator, or a left ventricular assist device within 5 years prior to the index admission were excluded. Patients who died during the index admission were also excluded from the study. Figure 1 outlines the cohort selection process.
Figure 1.
Study flow diagram. HF, heart failure; HFrEF, HF with reduced ejection fraction; non HFrEF, HF without reduced ejection fraction. Territories includes the Northwest Territories, Nunavut and Yukon.
Study variables
HFrEF
A simplified logistic model developed and validated by Uijl et al. was applied to differentiate between patients with HFrEF (ejection fraction < 40%) and those with HF without a reduced ejection fraction (ejection fraction ≥ 40%).13 A prediction threshold of 0.44 was used to maximize the specificity and sensitivity of the model.14 The variables incorporated in the Uijl model and their respective coefficients are provided in Supplemental Table S1.
Other medical history
Baseline patient characteristics were collected using demographic information at index hospitalization, and 6 months of hospitalization and mediation history. Comorbidity was summarized using the Charlson comorbidity index (CCI).14
Pharmacotherapy
Pharmacotherapy achieved by the 6-month follow-up period was evaluated based on the drugs and doses recommended by the CCS HF guideline (Supplemental Table S2).12 Pharmacotherapy treatment was classified as none, mono therapy (1 drug class), dual therapy (2 drug classes), or triple therapy (3 drug classes). The criteria for pharmacotherapy are defined in Supplemental Figure S1. In summary, any treatment with guideline-recommended HF medications (ACEi/ARB/ARNI, MRA, BB) was included if dispensed with supplies lasting ≥ 14 days post-index hospital discharge. If patients were on medication prior to index hospitalization, the medication was considered part of therapy if continued for ≥ 14 days postdischarge. Dual therapy was defined as 2 drug classes, each dispensed with supplies lasting ≥ 14 days and overlapping for ≥ 7 days. Triple therapy was defined as 3 drug classes, each dispensed with supplies lasting ≥ 14 days and overlapping for ≥ 7 days. A similar method was used by Deschaseaux et al., who also investigated treatment initiation patterns in HF. The overlap period used by Deschaseaux et al. was 14 days, vs the 7 days utilized in the current study.15 We found no statistical difference between an overlap period of 14 days vs 7 days in distinguishing between dual and triple therapy (Supplemental Table S3). Any patients that did not meet the above conditions were considered to not be on pharmacotherapy.
Vital status
Mortality status was assessed in 2 ways. The discharge disposition code in any subsequent hospitalization during the follow-up period was used to identify patients who died in-hospital. For these patients, the discharge date of the last hospitalization was recorded as the date of death. For patients who did not die during a subsequent hospitalization, we used the medication claims data. If a patient had no medication claims after a certain date, the last medication prescription date was recorded as the date of death. Patients with death dates preceding the 6-month follow-up date were considered to be dead at 6 months post-index discharge.
GDMT dosage and intensity
Dosage of medication was calculated as the proportion of the recommended target dose. The target doses for each HF medication are listed in Supplemental Table S2. Optimal GDMT was defined as receiving the following: ≥ 50% of the target dose for an ACEi, ARB, or ARNI; a BB; and any dose of an MRA.16
The intensity of pharmacotherapy was approximated using a GDMT scale adapted from Januzzi et al..17 Medication dosages were converted into the equivalent dose and summarized into a scaled score for each drug class (Supplemental Table S4). ACEis, ARBs, ARNIs, and BBs were scored from 0 to 5, and MRAs were scored from 0 to 4. The scores were added and summarized as a proportion of 14, the maximum achievable GDMT score (triple therapy: ACEi/ARB/ARNI + BB + MRA). The proportion of maximum achievable GDMT for all patients with HFrEF was calculated daily for the duration of 6 months, using medication dispensary data. This calculation was done by dividing the total daily intensity score for each patient by the maximum achievable intensity score of 14. Patients were then categorized into groups that achieved < 50%, 50%-74%, and ≥ 75% of the maximum achievable intensity scores, using either the last-day intensity score or the maximum intensity score during the 6-month period. The proportions of the maximum intensity score for all patients with HFrEF was averaged daily for 6 months post-HF hospitalization and plotted to observe the average trend of GDMT intensity for patients over the course of 6 months.
Statistical analysis
Categorical variables were summarized as count and percentage; continuous variables were summarized as mean and standard deviation (SD), or median with interquartile range, as appropriate.
The proportions of patients on GDMT and optimal GDMT each year were plotted from the 2013 and 2018 fiscal years, and the overall trend of change was analyzed using linear regression.
A logistic regression model was developed to identify factors associated with triple-therapy prescription among patients with HFrEF. The multivariable model controlled for sex, age, CCI, academic/community hospital type, urban/rural residence, income quintile, HF rehospitalization within 6 months of index discharge, and use of calcium channel blockers, hydrochlorothiazide, and other diuretics. We excluded 1023 patients (1.5%) with HFrEF with missing values for urban/rural residence, income quintile, and hospital type. Model results are presented as odds ratios with 95% confidence intervals (Cis).
Sensitivity analysis was conducted using a subset of the cohort alive at 6 months post-index discharge. Pharmacotherapy classification, GDMT dosage, and intensity were calculated using the alive cohort. All analyses were conducted using SAS Studio 3.8 (SAS Institute, Terry, NC).
Results
Patient characteristics
The study cohort consisted of 202,396 patients with incident HF hospitalization between October 2013 and September 2018. The mean age for the cohort was 81.3 years, and 47.9% were male (Table 1). Based on the Uijl model, 32.8% (n = 66,372) of the cohort had HFrEF. The median (interquartile range) CCI was 3.2, 3, 4
Table 1.
Patient demographics and clinical characteristics at index heart failure (HF) diagnosis
| Characteristic | All patients with HF (n = 202,396) | Patients with HFrEF (n = 66,372) |
|---|---|---|
| Age, y, mean (SD) | 81.3 (8.5) | 79.3 (8.1) |
| Male sex | 97,028 (47.9) | 51,180 (77.1) |
| Income quintile | ||
| 1 (lowest) | 53,092 (26.2) | 16,263 (24.5) |
| 2 | 45,518 (22.5) | 14,679 (22.1) |
| 3 | 39,251 (19.4) | 13,317 (20.1) |
| 4 | 32,922 (16.3) | 11,243 (16.9) |
| 5 (highest) | 29,928 (14.8) | 10,351 (15.6) |
| Residence type | ||
| Rural | 39,180 (19.4) | 13,536 (20.4) |
| Urban | 162,129 (80.1) | 52,521 (79.1) |
| Hospital type | ||
| Academic | 68,872 (34.0) | 23,364 (35.2) |
| Community | 133,517 (66.0) | 43,007 (64.8) |
| HFrEF | 66,372 (32.8) | 66,372 (100.0) |
| Alive 6 months post-index | 166,169 (82.1) | 55,882 (84.2) |
| HF rehospitalization within 6 months of index discharge | 45,356 (22.4) | 14,864 (22.4) |
| Comorbidities | ||
| Hypertension | 101,265 (50.0) | 24,578 (37.0) |
| Diabetes | 74,413 (36.8) | 27,239 (41.0) |
| Chronic obstructive pulmonary disease | 42,499 (21.0) | 10,005 (15.1) |
| Ischemic heart disease | 61,234 (30.3) | 27,329 (41.2) |
| Atrial fibrillation | 78,865 (39.0) | 19,183 (28.9) |
| Renal disease | 28,103 (13.9) | 8455 (12.7) |
| Charlson comorbidity index, median (IQR) | 3 (2–4) | 3 (1–4) |
| Medication history | ||
| ACEi/ARB | 89,082 (44.0) | 45,807 (69.0) |
| Beta blocker | 128,265 (63.4) | 59,461 (89.6) |
| MRA | 31,243 (15.4) | 19,955 (30.1) |
| Digoxin | 23,322 (11.5) | 9673 (14.6) |
| Diuretics | 151,468 (74.8) | 59,086 (89.0) |
| Calcium channel blockers | 81,885 (40.5) | 26,412 (39.8) |
| Hydrochlorothiazide | 24,954 (12.3) | 8648 (13.0) |
Values are n (%), unless otherwise indicated.
ACEi, angiotensin=converting enzyme inhibitor; ARB, angiotensin receptor blocker; HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; SD, standard deviation.
Medication use
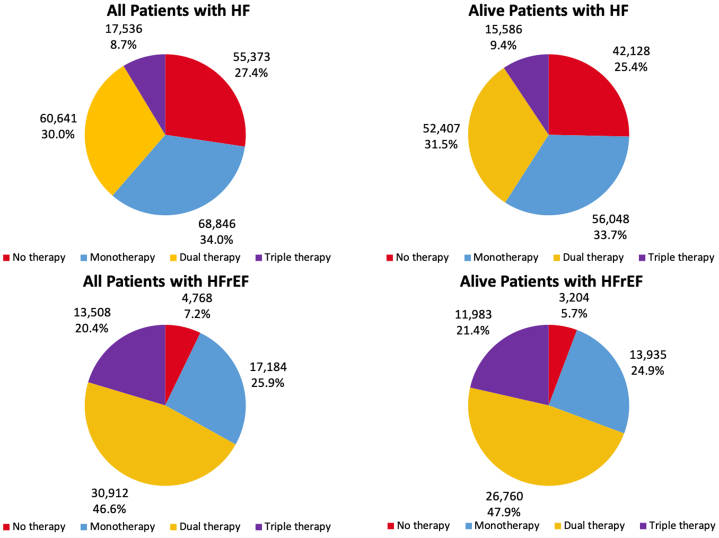
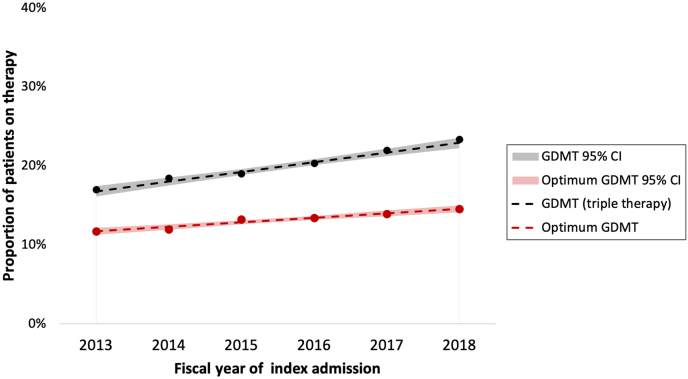
Among 66,372 patients with HFrEF, 13,508 (20.4%) were on triple therapy at any dose, 30,912 (46.6%) were on double therapy, 17,184 (25.9%) were on monotherapy, and 4768 (7.2%) were on no therapy (Fig. 2). Supplemental Table S5 provides details on specific drug classification dispensed to patients in each therapy group. Only 207 patients (1.5%) with HFrEF were on sodium-glucose transport protein 2 inhibitors (SGLT2is) and/or ivabradine. Of the total of 66,372 patients with HFrEF, only 8747 (13.2%) achieved optimal GDMT based on the maximum dose within 6 months. According to the last dispensed dose, only 5484 patients with HFrEF (8.3%) were on optimal GDMT 6 months postdischarge. Moreover, between 2013 and 2018, the proportion of patients on GDMT (triple therapy at any dose) increased an average of 1.2% (95% CI: 1.0%-1.4%) increase each fiscal year (P < 0.001). The average increase in the proportion of patients on optimal GDMT each fiscal year was 0.6% (95% CI: 0.4%-0.7%; P < 0.001).
Figure 2.
Pharmacotherapy achieved by patients with heart failure (HF) and those with HF with reduced ejection fraction (HFrEF) by 6 months post index discharge.
In the multivariable analysis of patients with HFrEF, women, patients who were treated in academic hospitals, and those who were rehospitalized within 6 months of their index discharge had higher odds of achieving triple therapy. Conversely, patients aged ≥ 80 years, those with more comorbidity, those residing in an urban setting, and those on calcium channel blockers or hydrochlorothiazide were less likely to receive triple therapy compared to their counterparts (Table 2).
Table 2.
Adjusted odds ratios (ORs) for being on triple therapy, compared to not being on triple therapy, for patients with heart failure with reduced ejection fraction
| Factor | Adjusted OR (95% CI) | P |
|---|---|---|
| Sex (ref: male) | ||
| Female | 1.52 (1.46–1.59) | < 0.0001 |
| Age (ref: 65–79), y | ||
| ≥ 80 | 0.58 (0.56–0.60) | < 0.0001 |
| Residence (ref: rural) | ||
| Urban | 0.96 (0.91–1.01) | 0.08 |
| Income quintile (ref: Lowest 1) | ||
| 2 | 1.07 (1.01–1.13) | 0.02 |
| 3 | 1.08 (1.02–1.14) | < 0.01 |
| 4 | 1.04 (0.98–1.10) | 0.24 |
| Highest 5 | 1.08 (0.98–1.11) | 0.16 |
| Hospital type (ref: community) | ||
| Academic | 1.15 (1.10–1.20) | < 0.0001 |
| Comorbidity | ||
| Higher CCI score | 0.91 (0.90–0.92) | < 0.0001 |
| Other diuretics (ref: no) | ||
| Yes | 1.69 (1.57–1.81) | < 0.0001 |
| Calcium channel blockers (ref: no) | ||
| Yes | 0.66 (0.64–0.69) | < 0.0001 |
| Hydrochlorothiazide (ref: no) | ||
| Yes | 0.79 (0.75–0.84) | < 0.0001 |
| Rehospitalized within 6 mo of index discharge (ref: no) | ||
| Yes | 1.41 (1.35–1.47) | < 0.0001 |
Multivariable logistic regressions were used.
CCI, Charlson Comorbidity Index; CI, confidence interval; HFrEF, heart failure with reduced ejection fraction; ref, referent.
Intensity of HF therapy
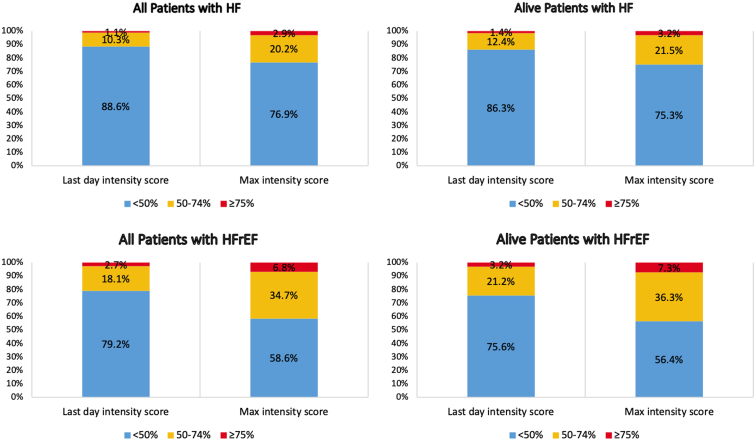
Of the patients with HFrEF, 38,869 (58.6%) achieved a score that was < 50% ( < 7 points) of the maximum intensity score (14 points), 23,006 (34.7%) achieved a score that was between 50% and 74% (7-10.4 points) of the maximum intensity score, and 4497 (6.8%) achieved a score that was ≥ 75% (≥ 10.5 points) of the maximum intensity score (Fig. 3). Observing the intensity score on the last day of the 6-month period, 52,572 (79.2%), 11,992 (18.1%), and 1808 (2.7%) patients had intensity scores that were < 50%, between 50% and 74%, and ≥ 75% of the maximum intensity score, respectively (Fig. 3).
Figure 3.
Categorization of patients with heart failure (HF) by proportion of maximum (max) guideline-directed medical therapy intensity score achieved. Intensity scores were calculated for each patient by starting with either the dose from the last day of therapy or the maximum therapy dose within 6 months post index, and then dividing by the maximum achievable intensity score. HFrEF, heart failure with reduced ejection fraction.
Including all patients with HF, and considering the peak dosage filled during the study period, 155,573 (76.9%) achieved a score that was < 50% of the maximum intensity score, 40,910 (20.2%) achieved a score that was between 50% and 74% of the maximum intensity score, and 5913 (2.9%) achieved a score that was ≥ 75% of the maximum intensity score (Fig. 3). Similarly, observing the intensity score on the last day of the 6-month period, 179,321 (88.6%), 20,777 (10.3%), and 2298 (1.1%) had intensity scores that were < 50%, between 50% and 74%, and ≥ 75% of the maximum intensity score, respectively (Fig. 3).
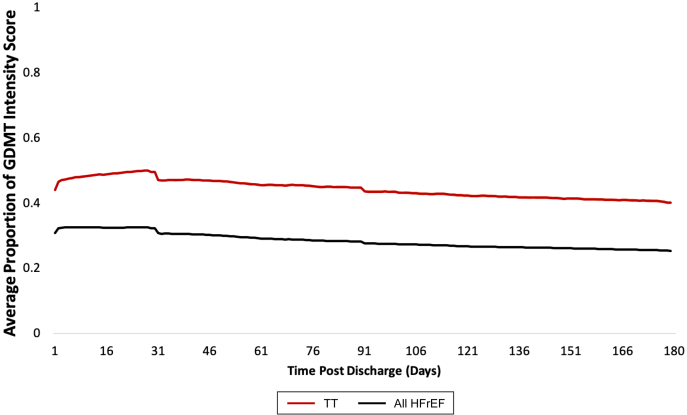
The mean proportion of the maximum intensity score for patients with HFrEF calculated daily over the 6-month postdischarge period is shown in Figure 4. For patients on triple therapy, the mean proportion of the maximum intensity score increased from 0.44 to 0.47 between day 1 and day 31, respectively. For all patients with HFrEF, the mean proportion of the maximum intensity score began at 0.31 on day 1 and continued to decline to a low of 0.25 on day 180 (Fig. 4).
Figure 4.
Average proportion of maximum guideline-directed medical therapy (GDMT) intensity score for patients with heart failure with reduced ejection fraction (HFrEF) calculated daily over 6 months post index discharge. Each data point is the average intensity score with 95% confidence interval for all patients with HFrEF (black, n = 66,372) or patients with HFrEF on triple therapy (TT; red, n = 13,508) divided by the maximum achievable Intensity score.
Sensitivity analysis
A sensitivity analysis was performed using the subcohort of patients with HF who were classified as having HFrEF and were considered alive at 6 months postdischarge (Fig. 2). Of the alive patients with HFrEF, 11,983 (21.4%) were on triple therapy, and 3204 (5.7%) did not receive any pharmacotherapy within 6 months following discharge from their index hospitalization (Fig. 2). Moreover, 7996 of alive patients with HFrEF (14.3%) achieved optimal GDMT based on the maximum dispensed dosage within 6 months, and 4989 (8.9%) were on optimal GDMT at 6 months postdischarge according to the last dispensed dose. The mean proportion of the maximum intensity score for alive patients with HFrEF increased from 0.43 to 0.48 between day 1 and day 31, and then declined to 0.44 on day 180 (Supplemental Fig. S2).
Discussion
In this national observational study of patients with incident HF-related hospitalization, we found that efforts to achieve optimal GDMT within 6 months of a hospitalization require greater attention. First, we identified that approximately one-fifth of patients with HFrEF are achieving triple therapy at either any dose or the optimal dose by 6 months after a HF hospitalization. Early initiation of optimal GDMT after index-HF hospitalization has proven to increase adherence and improve mortality outcomes.18 The exploration of the 6-month window allows for potential delays in care or further optimization of therapy, but it does not appear that this is occurring. Second, although patients with HFrEF achieved a higher intensity of pharmacotherapy compared to patients with HF without a reduced ejection fraction, more than half fail to achieve ≥ 50% of the maximal possible intensity score. A higher intensity of GDMT is associated with better outcomes.17 This study demonstrated that the gap in achieving optimal GDMT in patients with HFrEF remains wide, even in a publicly funded system with universal healthcare.
An externally validated model developed by Uijl et al. to identify patients with HFrEF using ICD-10 codes was used in the current study.13 Unless prescribed for comorbid conditions, the aforementioned medications have been shown to provide only morbidity and mortality benefit in patients with HFrEF.11 In our cohort, 32.8% of patients were identified as having HFrEF, which is similar to the percentages in reports from other HF cohorts.19 However, these results should be interpreted within the context of an understanding of the limitations of the model used for identifying potential patients with incident HFrEF. The simplified Uijl model had a specificity (accurate HFrEF prediction) of 83.1% for predicting an ejection fraction ≥ 40% when sensitivity and specificity is maximized using prevalence data.13 Compared to incident HF cohorts, prevalence HF cohorts have been shown to yield higher percentages of patients with HFrEF.20,21
Notably, 7.2% of patients with HFrEF in this study received no ACEi/ARB/ARNI, BB, or MRA in the 6 months posthospitalization, and 25.9% received only monotherapy. Our findings confirmed previous observations of suboptimal initiation of HF medications after HF diagnosis. For instance, 23.3% of patients with HFrEF did not receive any HF pharmacotherapy, and 22.1% received only monotherapy during the first year after diagnosis in the US,8 and this underutilization was shown to be linked to poorer outcomes.22 The current study observes data that predate the inclusion of SGLT2is and ivabradine into the CCS guidelines. Consequently, an inconsequential number of patients were on either medication and therefore were not included in the study.
Overall, 20.4% of patients with HFrEF were on triple therapy at any dose at 6 months of index hospitalization. Optimal GDMT, defined as receiving ≥ 50% of the target dose for ACEi/ARB/ARNI, and a BB, and any dose of an MRA, was achieved in only 13.2% of patients with HFrEF based on the maximum dispensed dosage within 6 months. The Guiding Evidence-Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) trial demonstrated similar results, with 15.5% of patients with HFrEF achieving optimal GDMT at 6 months.16 Even with biomarker-guided GDMT titration, many patients in the GUIDE-IT trial did not achieve optimal GDMT, a result that was attributed to patients being either clinically stable or already at maximally tolerated therapy.23 Similarly, medication data from the Change the Management of Patients With Heart Failure (CHAMP-HF) registry, which included outpatients with HFrEF in the US receiving ≥ 1 oral HF medication, also showed underutilization of HF medications, individually or in combination.9 In that study, only 1.0% of eligible patients were treated with triple therapy at target doses, and 22.1% of patients were treated with any dose of triple therapy.9
Reasons for underutilization of GDMT are likely multifactorial. Concordant with our findings, being older, having more comorbidities, and being in advanced New York Heart Association (NYHA) functional class III or IV have been reported as factors associated with less-intense medication titration. 1,24 These patients also are at the greatest absolute risk and often have similar outcomes on GDMT in clinical trials. Our study also demonstrates that patients on calcium channel blockers and hydrochlorothiazide are less likely to achieve GDMT. Although this study is observational in nature, the use of non-GDMT may coincide with precipitating side effects (ie, hypotension) that prevent initiation or uptitration of GDMT; consequently, physicians should prioritize GDMT over non-GDMT antihypertensives. Rehospitalization, on the other hand, increases the rate of GDMT usage in patients with HFrEF, which may indicate that the severity of condition is a justification for aggressive GDMT titration.
The GDMT intensity score data provide information on aggressiveness of dose titration within the 6 months after index hospitalization. In the current study, only 41.4% of patients with HFrEF achieved a score that was ≥ 50% of the maximal intensity score; however, patients with HFrEF are appropriately achieving a higher intensity of pharmacotherapy, compared to that for all patients with HF. The mean proportion of the maximum intensity score for all patients with HFrEF was calculated daily over 6 months postdischarge; in summary, it showed a gradual decline throughout the study period (Fig. 4). Notable periods in which a steeper decline in intensity scores appears are days 31 and 91, likely corresponding to time of medication refill, assessment for side effects, or intolerance by the physician. Nonetheless, a higher intensity of GDMT is associated with lower mortality rates17; therefore, the intensity scores and trends noted in the current study require significant improvement. However, the current study shows an overall yearly increase in the proportion of patients on GDMT, both at any does and at optimal dose, between 2013 and 2018 (Fig. 5). Translation of guidelines into clinical practice may take years, but the trend is reassuring.
Figure 5.
Trend of guideline-directed medical therapy (GDMT) between 2013 and 2018. Black: proportion of patients on triple therapy at any dose each fiscal year (%). Red: proportion of patients on optimum GDMT each fiscal year. Optimum GDMT defined as receiving ≥ 50% of the target dose for angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor, ≥ 50% of the target dose for beta blocker, and any dose of mineralocorticoid receptor antangonist. CI, confidence interval.
The strengths of the current study include the use of a large sample size from a representative cohort in a universal healthcare system, thereby mitigating the effect of interprovincial variables and establishing generalizable results. The current study also provides insight into prescription and adherence patterns in single-payer, largely public healthcare systems. Previous studies, by contrast, have looked mainly at data from the US, a multi-payer, heavily privatized system. The focus on patients aged ≥ 65 years also removes variables such as drug affordability, which may affect the likelihood of filling prescriptions, as universal drug coverage is available to this age cohort in Canada. We infer that prescribing patterns, however, should not change for those aged < 65 years.
The study also has potential limitations. As mentioned, the cohort is limited to patients aged ≥ 65 years; therefore, the results may not be entirely generalizable to the younger HFrEF population. In an epidemiologic study of patients in Australia with HFrEF aged ≥ 45 years, 42.3% were aged between 45 and 64 years.25 The fact that the study is observational means it has potential to have unmeasured confounders. The lack of echocardiography-based left ventricular ejection fraction data, and utilization of an administrative data-based model to predict left ventricular ejection fraction in patients with HF, may result in misclassification bias. Moreover, due to the lack of out-of-hospital mortality data, we assumed that those without any prescription dispensed during the follow-up period were deceased. Finally, the analysis utilizes records of medications that were dispensed, but it does not include prescriptions that were not filled, nor does it take into account whether they were taken as prescribed.
Conclusion
Efforts to achieve optimal GDMT in patients with HFrEF post-index HF-related hospitalization remain suboptimal. Current clinical practice, where optimal pharmacologic management of HFrEF falls short, does not align with the existing evidence that supports aggressive titration of GDMT post-HF diagnosis. Considering the observed gap in care, further studies are required to investigate innovative strategies to optimize the HF care in this patient population.
Clinical perspectives
The current study outlines the care gaps evident in the treatment of patients with HFrEF. GDMT has significant morbidity and mortality benefits; unfortunately, current practice fails to initiate and titrate medications effectively. Solutions to improve GDMT postdischarge include more frequent outpatient appointments scheduled at the time of discharge (ie, every 4-6 weeks where possible); lack of follow-up appointments may explain some of the issues with slow titration. Clinicians should also prioritize GDMT over use of non-GDMT antihypertensives when initiating and titrating medications.
Translational outlook
GDMT initiation and titration in patients with HFrEF remain suboptimal. Further studies are required to determine strategies to optimize GDMT therapy in these patients. Research should focus on determining causal factors that influence poor GDMT prescribing patterns and establishing solutions to counteract these problems.
Acknowledgements
Parts of this material are based on data and information provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not those of the Canadian Institute for Health Information. P.K. holds a Canadian Institutes of Health Research (CIHR) Sex and Gender Science Chair and a Heart and Stroke Foundation Chair in Cardiovascular Research.
Funding Sources
The study was supported by funds received as part of P.K.’s Heart and Stroke Foundation Chair. The foundation had no input into study design, analysis, or interpretation.
Disclosures
J.A.E. reports research support for trial leadership from Bayer, Merck & Co, Novo Nordisk, Cytokinetics, Applied Therapeutics, American Regent; honoraria for consultancy from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Otsuka, Bayer, Merck & Co, Novartis; and serves as an advisor to US2.ai. The additional authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study was approved by the University of Alberta Research Ethics Board (Pro00040008).
See page 1022 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.08.003.
Supplementary Material
References
- 1.Jarjour M., Henri C., de Denus S., et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8:725–738. doi: 10.1016/j.jchf.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer M.A., McMurray J.J.V., Velazquez E.J., et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 3.McMurray J.J.V., Packer M., Desai A., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 4.Packer M., Fowler M.B., Roecker E.B., et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B., Zannad F., Remme W.J., et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 6.Cole G.D., Patel S.J., Zaman N., et al. “Triple therapy” of heart failure with angiotensin-converting enzyme inhibitor, beta-blocker, and aldosterone antagonist may triple survival time. Shouldn’t we tell patients? JACC Heart Fail. 2014;2:545–548. doi: 10.1016/j.jchf.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Vaduganathan M., Claggett B.L., Jhund P.S., et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128. doi: 10.1016/S0140-6736(20)30748-0. [DOI] [PubMed] [Google Scholar]
- 8.Wirtz H.S., Sheer R., Honarpour N., et al. Real-world analysis of guideline-based therapy after hospitalization for heart failure. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene S.J., Butler J., Albert N.M., et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 10.Savarese G., Bodegard J., Norhammar A., et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden) Eur J Heart Fail. 2021;23:1499–1511. doi: 10.1002/ejhf.2271. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz J.A., O’Meara E., McDonald M.A., et al. 2017 Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 12.McDonald M., Virani S., Chan M., et al. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. 2021;37:531–546. doi: 10.1016/j.cjca.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Uijl A., Lund L.H., Vaartjes I., et al. A registry-based algorithm to predict ejection fraction in patients with heart failure. ESC Heart Fail. 2020;7:2388–2397. doi: 10.1002/ehf2.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H., Vijaya S., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.Deschaseaux C., Mcsharry M., Hudson E. Treatment initiation patterns, modifications, and medication adherence among newly diagnosed heart failure patients: a retrospective claims database analysis. J Manag Care Spec Pharm. 2016;22:561–571. doi: 10.18553/jmcp.2016.22.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiuzat M., Ezekowitz J., Alemayehu W., et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT trial: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2020;5:757–764. doi: 10.1001/jamacardio.2020.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Januzzi J.L., Jr., Ahmad T., Mulder H., et al. Natriuretic peptide response and outcomes in chronic heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;74:1205–1217. doi: 10.1016/j.jacc.2019.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gayat E., Arrigo M., Littnerova S., et al. Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity-score matched study. Eur J Heart Fail. 2018;20:345–354. doi: 10.1002/ejhf.932. [DOI] [PubMed] [Google Scholar]
- 19.Tsao C.W., Lyass A., Enserro D., et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savarese G., Vasko P., Jonsson Å., et al. The Swedish Heart Failure Registry: a living, ongoing quality assurance and research in heart failure. Ups J Med Sci. 2019;124:65–69. doi: 10.1080/03009734.2018.1490831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brugts J.J., Linssen G.C.M., Hoes A.W., Brunner-La Roca H.P., CHECK-HF investigators Real-world heart failure management in 10,910 patients with chronic heart failure in the Netherlands: design and rationale of the Chronic Heart failure ESC guideline-based Cardiology practice Quality project (CHECK-HF) registry. Netherlands Heart J. 2018;26:272–279. doi: 10.1007/s12471-018-1103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilstrap L.G., Fonarow G.C., Desai A.S., et al. Initiation, continuation, or withdrawal of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felker G.M., Anstrom K.J., Adams K.F., et al. Effect of natriuretic peptide—guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318:713–720. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng T.H.K., Tromp J., Tay W.T., et al. Prescribing patterns of evidence-based heart failure pharmacotherapy and outcomes in the ASIAN-HF registry: a cohort study. Lancet Glob Heal. 2018;6:e1008–e1018. doi: 10.1016/S2214-109X(18)30306-1. [DOI] [PubMed] [Google Scholar]
- 25.Chan Y.K., Tuttle C., Ball J., et al. Current and projected burden of heart failure in the Australian adult population: a substantive but still ill-defined major health issue. BMC Health Serv Res. 2016;16:1–10. doi: 10.1186/s12913-016-1748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.