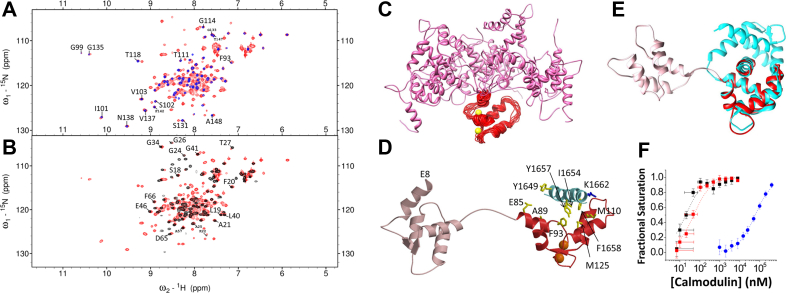
Figure 4.
NMR-derived structures of Ca2/CaM12’-IQ. A, 15N-1H HSQC NMR spectrum of 15N-labeled Ca2/CaM12’ bound to unlabeled IQ (red) is overlaid with the spectrum of Ca2+-bound CaMWT C-lobe/IQ complex (blue). B, NMR spectrum of 15N-labeled Ca2/CaM12’ bound to unlabeled IQ (black) is overlaid with the spectrum of Ca2+-free CaM12’ (red). C, ensemble of 10 lowest energy NMR structures of Ca2/CaM12’ (PDB ID: 7L8V). Main chain structures are depicted by a ribbon diagram. Structures of the C-lobe (residues 85–149) are overlaid and highlighted in red; N-lobe structures (residues 1–84) are highlighted in pink. Bound Ca2+ ions are yellow. Structural statistics are given in Table 2. D, the lowest energy structure of Ca2/CaM12’-IQ complex is shown as a ribbon diagram of Ca2/CaM12’ bound to the IQ peptide (cyan). The CaM N-lobe and C-lobe are highlighted pink and red, respectively. Side-chain atoms of key residues are depicted by sticks and are colored yellow and blue. E, overlay of the NMR structure of Ca2/CaM12’-IQ (C-lobe in red) with the crystal structure of Ca4/CaM (cyan, 2BE6). The C-lobe structures overlay with an RMSD of 1.8 Å. F, fluorescence polarization assay showing the binding of half Ca2+-saturated CaM mutant (Ca2/CaM12’) with fluorescently labeled IQ peptides (WT: black; K1662E: red; both: KD < 100 nM), and of apoCaM binding to Y1657D (blue, KD = 60 μM). CaM, calmodulin; PDB, Protein Data Bank.