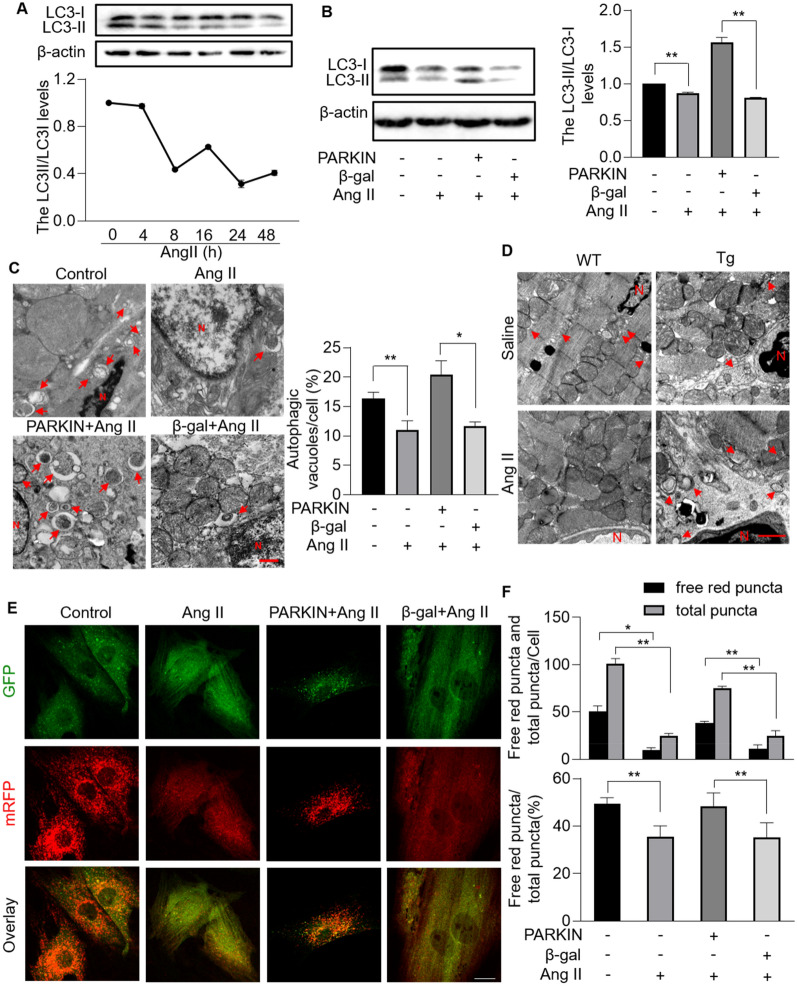
Fig. 3.
Mitophagy was impaired in Ang II-treated cardiomyocytes and hearts, which was rescued by overexpression of PARKIN. A, Immunoblotting results showing the protein levels of light chain 3 (LC3) I and LC3 II in cardiomyocytes exposed to Ang II at the indicated time. n = 3 experiments per group. The ratio of LC3II/LC3I was calculated. B, Enforced expression of PARKIN rescued Ang II-induced decreased LC3II/LC3I ratio. Cardiomyocytes infected with Parkin adenovirus or β-gal adenovirus were exposed to Ang II. Immunoblot was performed to detect protein levels of LC3I and LC3II (left). n = 3 experiments per group. The ratio of LC3II/LC3I was calculated (right). ** p < 0.01. C and D, PARKIN restored autophagic vacuoles in hypertrophic model. Autophagic vacuoles were visualized in cardiomyocytes infected with Parkin adenovirus or β-gal (C, left; bar = 500 nm), and hearts of Parkin transgenic mice or WT mice (D; bar = 1 µm). Quantitation of autophagic vacuoles were shown in (C, right). * p < 0.05. ** p < 0.01. n = 3 experiments per group. E and F, Overexpression of PARKIN attenuated Ang II-induced mitophagy flux defects. LC3 adenovirus tandem-labeled green fluorescent protein (GFP)-monomeric red fluorescent protein (mRFP) (GFP-mRFP-LC3) was used to indicate mitophagy flux. GFP-mRFP-LC3 was expressed and detected in 24 h after transfection in cardiomyocytes with overexpression or PARKIN or not. GFP-LC3 (green puncta), mRFP (red puncta, representative of autolysosomes) and overlay (yellow puncta, representative of autophagosomes) (E; bar = 25 µm). Quantification results were shown in (F). * p < 0.05. ** p < 0.01. n = 3 experiments per group