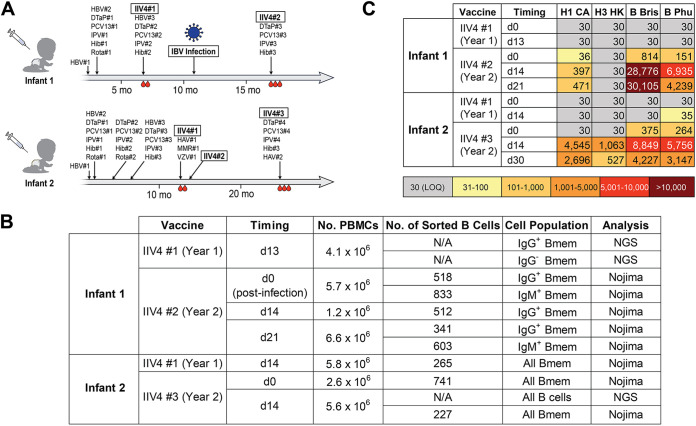
FIG 1.
Study design and sample description. (A) Immunization and influenza infection history for the two infants enrolled in the study. Blood drop symbols indicate the blood draws analyzed in the study. HBV, hepatitis B vaccine; DTaP, diphtheria, tetanus, and pertussis vaccine; PCV, pneumococcal vaccine; IPV, polio vaccine; Hib, Haemophilus influenzae type B vaccine; Rota, rotavirus vaccine; IIV4, quadrivalent inactivated influenza vaccine; HAV, hepatitis A virus vaccine; MMR, measles, mumps, and rubella vaccine; VZV, varicella-zoster virus vaccine. (B) Summary of B cells analyzed in this study. (C) Plasma IgG binding titers against HAs included in the IIV4. Values indicate ED50s from ELISA. H1 CA, A/California/2009 X181; H3 HK, A/Hong Kong/2014 X263B; B Bris, B/Brisbane/60/2008; B Phu, B/Phuket/3073/2013; LOQ, limit of quantitation.