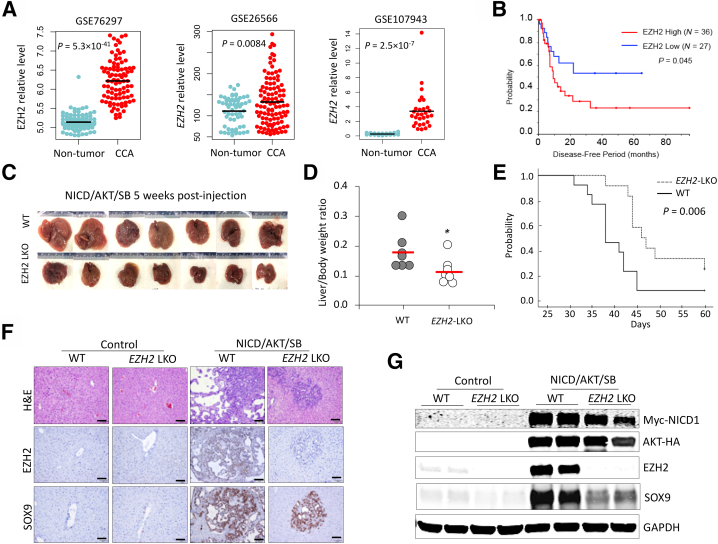
Figure 1.
Liver-specific deletion of enhancer of Zeste homolog 2 (EZH2) inhibits development of cholangiocarcinoma (CCA) in mice. A: EZH2 expression in human CCAs and non-tumorous tissues from Gene Expression Omnibus data sets GSE76297 (containing 91 CCAs and 92 adjacent nontumor samples) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE76297), GSE26566 (containing 104 CCAs, 59 matched nontumor samples, as well as six normal intrahepatic bile duct samples) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE26566), and GSE107943 (containing 30 CCAs and 27 matched nontumor samples) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107943). B: Kaplan-Meier survival and log-rank test showing the disease-free survival periods of patients with CCA in TCGA-CHOL and GSE107943 data sets. Patients were assigned to EZH2 high or EZH2 low groups with the median EZH2 level of each cohort as cutoff. C and D: Intrahepatic CCAs in liver-specific EZH2 knockout (EZH2-LKO) mice and control mice (C57BL/6 background) induced by hydrodynamic injection of Notch1 intercellular domain (NICD), AKT, and Sleeping Beauty (SB) transposase plasmids. Gross images of livers of each group at 5 weeks postinjection are shown in C, and the liver/body weight ratios of these mice are shown in D. E: Kaplan-Meier survival and log-rank test of EZH2-LKO and littermate control (wild-type [WT]) mice injected with NICD/AKT/SB plasmids (n = 12 per group). F and G: Hematoxylin and eosin (H&E) and immunohistochemistry stains (F), and Western blot analysis for indicated molecules (G) in the liver/tumor tissues from NICD/AKT/SB–injected mice and noninjected control mice (as shown in C). Data are expressed as means ± SD. ∗P < 0.05. Scale bars = 100 μm. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.