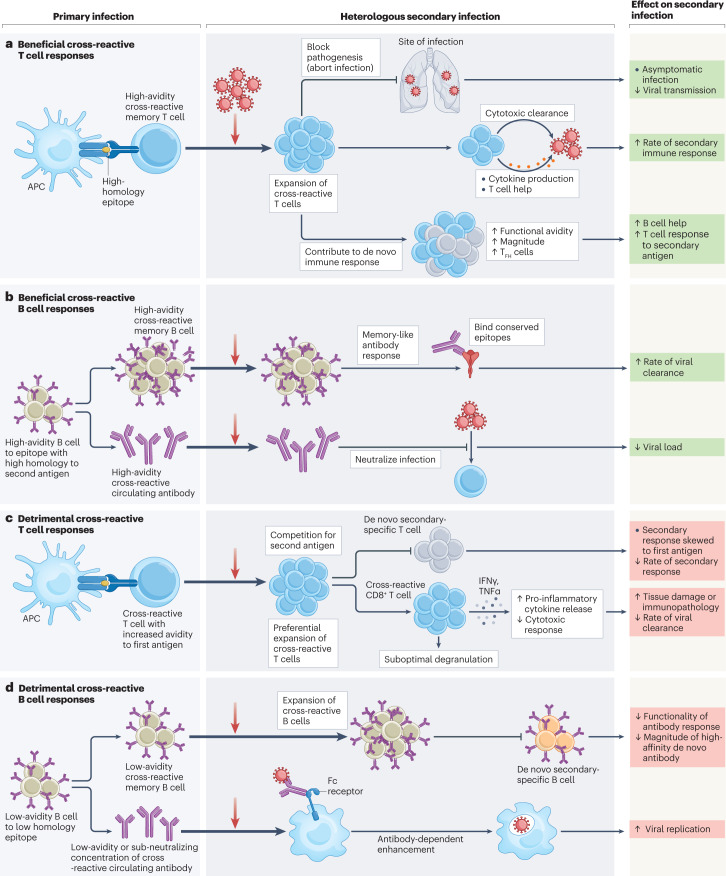
Fig. 1. Mechanisms of beneficial or detrimental cross-reactive immune responses identified in heterologous secondary viral infections.
Cross-reactive T and B cells may be generated in a primary infection with a virus that has sequence similarity to a heterologous secondary infectious agent. Cross-reactive immune cells may have beneficial or detrimental effects, and the mechanisms for such effects have been determined for many different virus families. a, Beneficial cross-reactive T cells may be generated through the priming of T cells by high-homology epitopes. These T cells then cross-react with high avidity during a heterologous secondary infection and may block pathogenicity by preventing invasive infection (aborting infection) or may expedite the rate of viral clearance by forming a ‘secondary-like’ memory immune response with an increased magnitude of B cell help and T cell responses. Evidence for such cross-reactive mechanisms was reported in studies of coronavirus infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)39,62,90,100. b, Beneficial cross-reactive memory B cells and antibodies can be generated to epitopes of high similarity between a primary and a heterologous secondary infection. Cross-reactive B cells specific to highly conserved epitopes may then go on to produce an expedited and high-functionality memory-like response to heterologous infection, including the production of cross-neutralizing antibodies that can prevent viral entry to cells. Evidence for these cross-reactive mechanisms was reported in coronavirus infections, including Betacoronaviruses and SARS-CoV-2 (refs. 49,108), influenza infection109 and influenza vaccination110 studies. c, Cross-reactive T cells generated to epitopes of relatively low homology may detrimentally impact a secondary infection with heterologous agents by dominating the response to the secondary infection, competing for presented antigen and disrupting the production of high-avidity de novo T cell responses. Cross-reactive T cells may bind with lower affinity to epitopes from the secondary infectious agent compared with the first. Low-affinity epitope binding by cross-reactive CD8+ T cells may then lead to the production of pro-inflammatory cytokines (interferon-γ (IFNγ), tumour necrosis factor-α (TNFα)) without the induction of cytolytic effector functions (degranulation) — leading to immunopathology and reducing viral clearance. Evidence for these cross-reactive mechanisms has been reported in studies of flavivirus infection36,111,112, reviewed elsewhere113,114. d, Cross-reactive B cells generated to epitopes of low homology may interfere with responses to a secondary heterologous infection by skewing the response to antibodies with higher avidity for the initial infectious agent, reducing the generation of de novo antibody responses. Evidence for such cross-reactive mechanisms was reported in coronavirus (common cold coronaviruses and SARS-CoV-2)85 and influenza virus studies, reviewed elsewhere115. Low-avidity cross-reactive antibodies or low levels of high-avidity cross-reactive antibodies that cannot neutralize a secondary heterologous infection may bind to virus and aid viral entry into cells through Fc-receptor-mediated endocytosis, a mechanism known as antibody-dependent enhancement. Evidence for antibody-dependent enhancement was reported in studies of flavivirus infection116,117. APC, antigen-presenting cell; TFH cell, follicular helper T cell.