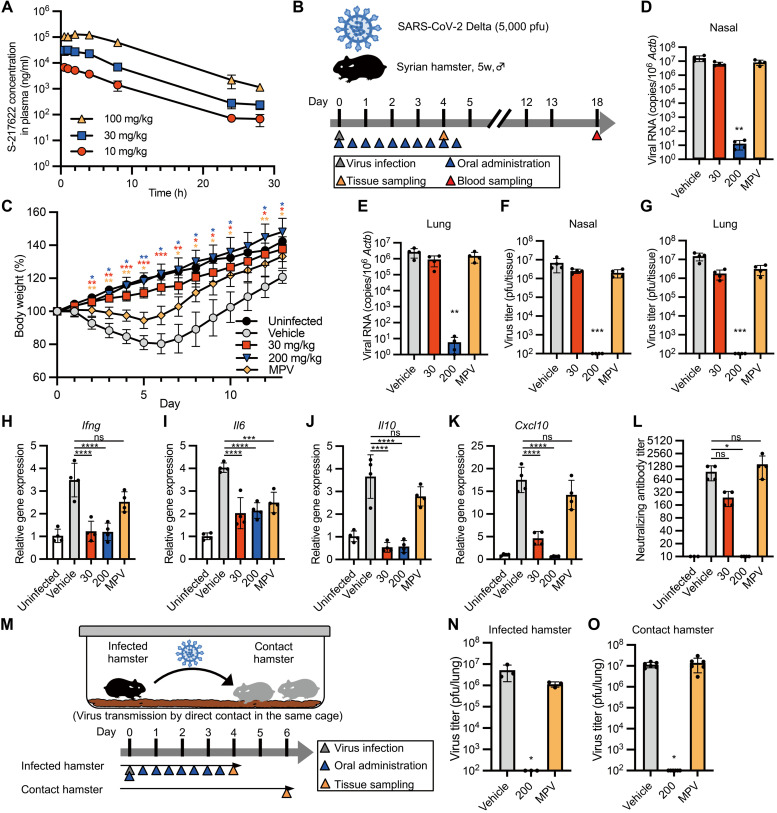
Fig. 2. Prophylactic treatment of S-217622 controls viral burden and disease in hamsters inoculated with SARS-CoV-2.
(A)The plasma concentration profile of S-217622 after a single oral administration in hamsters is shown (n = 3 for each treatment). Plasma samples were harvested at the indicated time points and analyzed by LC-MS/MS. (B) Schematic of the experimental design for prophylactic treatment in a hamster model. Hamsters were intranasally inoculated with 5,000 pfu of SARS-CoV-2 Delta variant. For prophylactic treatment, the hamsters were treated with oral administration of S-217622 or vehicle (0 mg/kg) twice daily (b.i.d.) from the time of infection (0 hpi) to 4 dpi. Molnupiravir (MPV) was used as a comparator drug. A group of hamsters (n = 4 for each treatment) was euthanized at 4 dpi for tissue collection. Another subset of hamsters (n = 4 for each treatment; n = 3 for uninfected) was monitored for 13 days for body weight change and then euthanized at 18 dpi for serum collection. (C) Body weight changes in uninfected hamsters (n = 3) and SARS-CoV-2-infected hamsters treated with S-217622 (30 mg/kg and 200 mg/kg), vehicle, or MPV (200 mg/kg) is shown (n = 4 for each group). (D and E) Viral RNA concentrations were measured in nasal turbinates (D) and lungs (E) isolated from hamsters at 4 dpi. Each group of hamsters was treated with vehicle (red), 30 mg/kg (blue), and 200 mg/kg (orange) of S-217622 or MPV (green) (n = 4 for each group). Relative viral RNA abundance in lungs as compared with lungs from vehicle-treated hamsters were examined. Data were normalized to β-actin. (F and G) Virus titers were measured in nasal turbinates (F) and lungs (G) isolated from hamsters at 4 dpi as determined by plaque assay. Each group of hamsters was treated with vehicle (red), 30 mg/kg (blue) and 200 mg/kg (orange) of S-217622, or MPV (green) (n = 4 for each group). (H to K) Cytokine gene expression was measured in lungs isolated from hamsters at 4 dpi (n = 4 for each group). Relative gene expression of Ifng (H), Il6 (I), Il10 (J), and Cxcl10 (K) in the lungs was compared to lungs from uninfected hamsters. Data were normalized to Actb. (L) Neutralizing antibody titers were measured in hamster serum at 18 dpi. (M) A schematic of the experimental design for the virus transmission experiment is shown. One hamster per cage was inoculated with 5,000 pfu of SARS-CoV-2 Delta variant (infected hamster). Two naïve hamsters (contact hamsters) were co-housed with the infected hamster. Only the infected hamsters were prophylactically treated with S-217622 or MPV from the time of infection (0 hpi) (b.i.d.) Each treatment group consists of three infected hamsters and six contact hamsters in three cages. (N and O) Virus titers were measured in lungs isolated from the infected hamsters at 4 dpi (N) and isolated from the contact hamsters at 6 days after co-housing (O). Each group of the infected hamsters was treated with vehicle (red), 200 mg/kg (blue), or MPV (green). The values shown are mean ± SD. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by two-way ANOVA with Dunnett’s test (C), Kruskal-Wallis test with Dunn’s test (D to G, L, N, O) or one-way ANOVA with Tukey’s test (H to K).