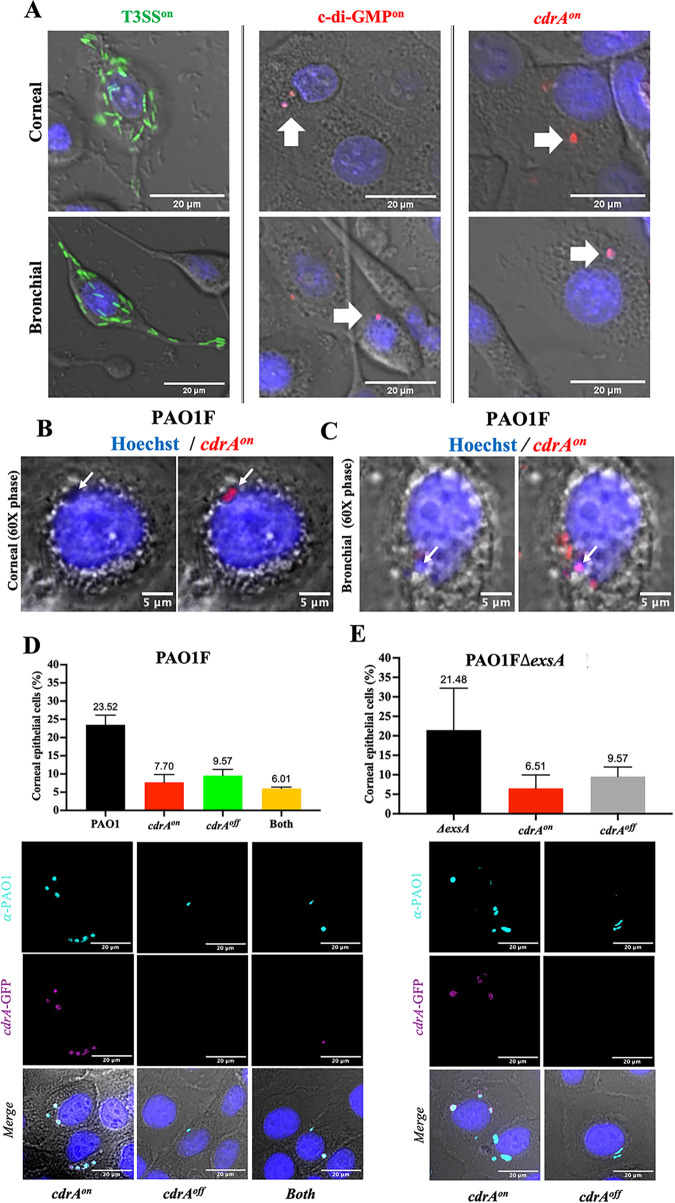
FIG 1.
Intracellular wild-type P. aeruginosa form distinct phenotypes within human epithelial cells. (A) Intracellular expression of genes encoding the T3SS (green), chronic switch regulator c-di-GMP (red), or biofilm matrix protein CdrA (cdrA-GFP, pseudocolored red) in human corneal or bronchial epithelial cells at 6 h postinfection with P. aeruginosa PAO1F. Extracellular bacteria were killed at 3 h postinfection with amikacin (200 μg/mL). T3SS-expressing bacteria occupied the cytosol as expected. In contrast, bacteria reporting c-di-GMP or cdrA expression formed distinct localized vacuole-like niches within the same or different cells. (B and C) Oil-immersion phase-contrast microscopy shows cdrA-GFP-reporting bacteria (pseudocolored red puncta) of wild-type PAO1F localized to vacuoles in corneal epithelial cells (B) or bronchial epithelial cells (C). (D) Percentage of corneal epithelial cells containing any intracellular P. aeruginosa (PAO1F, black bar) and distinct cdrA-expressing phenotypes. After labeling all bacteria with anti-Pseudomonas antibody, cdrA-GFP reporter expression allowed subdivision of invaded cells into those only containing cdrAon bacteria (red bar), only cdrAoff (green bar), and both cdrAon and cdrAoff (yellow bar). (E) Percentage of corneal epithelial cells containing intracellular PAO1FΔexsA (black bar), a T3SS mutant that only localizes to vacuoles (9). Cells containing exsA mutants showed a vacuolar distribution similar to that of the wild type with some only containing cdrAon (red bar) and others containing cdrAoff vacuolar bacteria (gray bar). In panels D and E, there was no significant difference between subgroups in the distribution of vacuolar phenotypes (one-way ANOVA with Dunnett’s multiple-comparison test). Data are shown as the mean ± SD of three biological replicates. Images below the quantitative data in panels D and E show examples of corneal epithelial cells for each of the categories quantified (bacteria pseudocolored as indicated: cdrA-GFP, magenta; anti-Pseudomonas antibody, cyan).