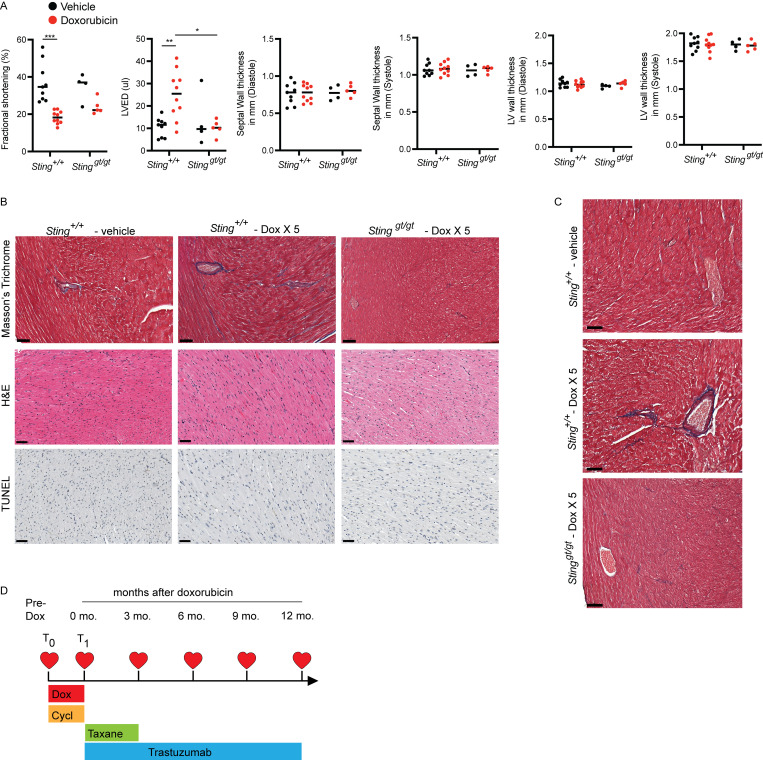
Figure S4.
Functional and histologic characterization of cardiac toxicity after doxorubicin. (A) Echocardiogram results from mice of indicated genotypes 14 d after completing 5 wk doses of doxorubicin or vehicle. N = 9–10 for Sting+/+ mice and N = 4–5 for Stinggt/gt mice; two independent experiments were performed. *P < 0.05, **P < 0.01, ***P < 0.0001, two tailed T test. (B). Masson’s trichrome, H&E, and TUNEL stains of the left ventricle of indicated genotype 2 wk after completing five doses of doxorubicin or vehicle treatment only; scale bars = 50 μm. Fibrosis was evident in doxorubicin treated Sting+/+ mice, but no evidence of apoptosis was apparent. Experiment performed with N = 4 mice for each treatment condition and timepoint; representative image shown. (C) Masson’s trichrome stain of the left ventricle in mice of indicated genotype 10 mo after completing five doses of doxorubicin or vehicle treatment only; scale bars = 50 μm. N = 3–4 for each condition; representative image shown. (D) Study schema for MSKCC IRB 14-099, a clinical trial to identify biomarkers of cardiac toxicity in HER2+ breast cancer patients receiving doxorubicin-based polychemotherapy and trastuzumab. Timepoints of echocardiograms and blood biospecimens obtained on the trial in relation to the treatment schedule. T0 denotes pre-treatment timepoint, and T1 denotes a post-doxorubicin, pre-trastuzumab timepoint.