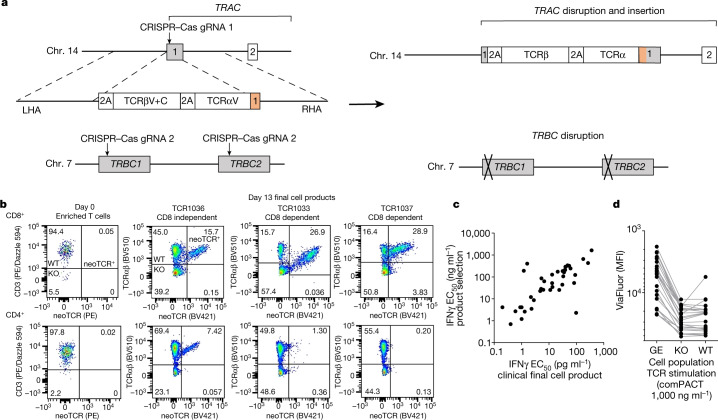
Fig. 2. Non-viral precision genome-engineering for clinical-grade cell manufacture.
a, Schematic of the construct design and resulting editing. b, Examples of endogenous TCR knockout and knock-in of up to three neoTCRs in a final clinical-grade cell product. Day 0 (left column) shows an example of the same patient’s enriched T cell product but was not stained with the peptide–HLA multimer. Day 13 flow plots (right 3 columns) show the results of each of the 3 neoTCR product lots for that patient. c, TCR functionality (potency) as evaluated by IFNγ production correlates between small-scale products generated from healthy donor T cells and the final large-scale clinical-grade cell product. The functionality of the neoTCR clinical-grade product made for patient autologous cells (IFNγ EC50 values measured by ELISA or ELLA Simple Plex) was correlated with the functionality of the neoTCR product made in healthy donor cells at product selection (IFNγ EC50 values measured by CBA; Pearson’s r = 0.8412, P < 0.0001). d, Proliferation analysis of the final neoTCR clinical-grade cell product following exposure to peptide–HLA stimulation at 1,000 ng ml–1. Each dot represents a unique neoTCR product. KO, knockout of the endogenous TCR only, these cells do not have a TCR on their surface; MFI, mean fluorescence intensity; neoTCR+ or GE, gene-edited knockout of wild-type TCR and knock-in of neoTCR; WT, wild-type, unedited cells expressing the endogenous TCR.