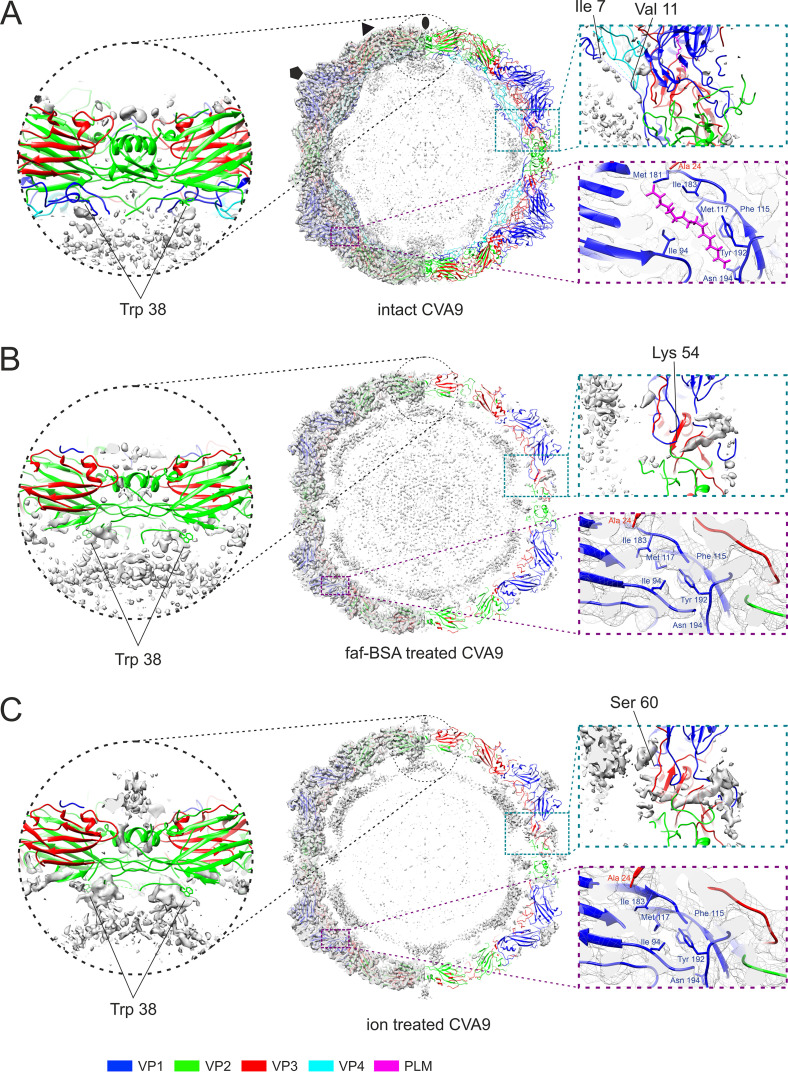
FIG 7.
Comparison of reconstructions and atomic models of intact, 0.01% faf-BSA-treated, and endosomal ion-treated CVA9 virions. (A) Untreated CVA9 central section through the model fitted to the reconstruction shown at 3 σ above mean. (Left) Cryo-EM density with atomic model fit; (right) cryoEM density accounted for by the atomic model (not shown) to reveal the unmodeled density. Symmetry axes are marked as follows: 5-fold, pentagon; 3-fold, triangle; 2-fold, ellipse. Enlarged view of the model at a 2-fold symmetry axis highlights the vRNA contacts with Trp38 from VP2, observed in all three reconstructions. The enlarged view on the right shows that most of the density in the capsid region was accounted for. The VP1 N terminus extends on the inside of the capsid hugging VP4, and density for a lipid factor can accommodate an atomic model of a palmitate. (B) Central section through the atomic model fitted into the reconstruction of 0.01% faf-BSA-treated CVA9. On the right, half of the density that was accounted by an atomic model was removed to better visualize the unmodeled density. Enlarged view of vRNA contacts with Trp38 from VP2 is shown. View of unmodeled density near the VP1 N terminus (Lys54) spanning the capsid of expanded virions and collapsed hydrophobic pocket are zoomed on the right. (C) Central section through the atomic model fitted into the reconstruction of endosomal ion-treated CVA9. On the right, half of the density that was accounted by an atomic model was removed to better visualize the unmodeled density. Enlarged view of vRNA contacts with Trp38 from VP2 is shown, in which the density above 2-fold can also be seen. View of unmodeled density near the VP1 N terminus (Ser60) spanning the capsid of expanded virions and collapsed hydrophobic pocket are zoomed on the right.