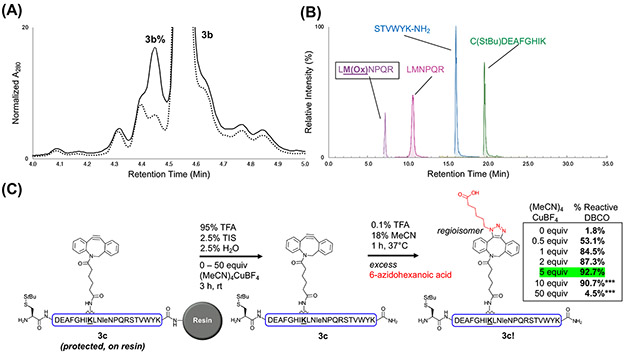
Figure 3:
Assessment of the (MeCN)4CuBF4 DBCO protection strategy’s robustness. Peptide 3b (all canonical residues represented) was used to determine the strategy’s oxidation potential, and peptide 3c (Met-to-Nle variant of 3a) was used to establish effective amounts of Cu(I) salt to use for DBCO protection. (A) Overlaid LC/MS chromatogram showing 3b cleaved with (solid line) or without (dashed line) ~3 equiv (MeCN)4CuBF4. Integration of the labeled peak areas suggested oxidation (3b%) increased from 6% to 12.5% with Cu(I) salt addition to the standard cleavage cocktail. LC/MS method C was used, and the individual chromatograms are shown in Fig. S11. (B) Trypsin digestion and LC/MS/MS analysis of 3b revealed Met as the oxidation-susceptible residue. The 3b sequence is color-coded by trypsin-digested fragments and matches the appropriate ion signal identified through MS/MS analysis. LM(Ox)NPQR (far left peak, purple) indicates peptide containing oxidized Met. (C) Cleavage of 3c with different equiv of (MeCN)4CuBF4 indicated that 5 equiv provides the most effective DBCO protection (green). After performing SPAAC on crude 3c, A280 peak areas of reacted (3c!) and unreacted peptide were integrated to calculate % reactive DBCO. ***Indicates higher equiv of (MeCN)4CuBF4 that were found to complex with the DBCO peptide, preventing reaction with azide after cleavage. Although the % reactive DBCO is lower, this decrease is not due to 5-endo-dig-cycloisomerization. Representative LC/MS chromatograms of crude 3c (before and after SPAAC) are shown in Fig. S15-17.