Abstract
Background
Solid organ transplant (SOT) recipients are at increased risk for morbidity and mortality from COVID-19 due to their immunosuppressed state and reduced immunogenicity from COVID-19 mRNA vaccines. This investigation examined the association between COVID-19 mRNA vaccination status and mortality among SOT recipients diagnosed with COVID-19.
Methods & findings
A retrospective, registry-based chart review was conducted investigating COVID-19 mortality among immunosuppressed solid organ transplant (SOT) recipients in a large metropolitan healthcare system in Houston, Texas, USA. Electronic health record data was collected from consecutive SOT recipients who received a diagnostic SARS-CoV-2 test between March 1, 2020, and October 1, 2021. The primary exposure was COVID-19 vaccination status at time of COVID-19 diagnosis. Patients were considered ‘fully vaccinated’ at fourteen days after completing their vaccine course. COVID-19 mortality within 60 days and intensive care unit admission within 30 days were primary and secondary endpoints, respectively. Among 646 SOT recipients who were diagnosed with COVID-19 at Houston Methodist Hospital between March 2020, and October 2021, 70 (10.8%) expired from COVID-19 within 60 days. Transplanted organs included 63 (9.8%) heart, 355 (55.0%) kidney, 108 (16.7%) liver, 70 (10.8%) lung, and 50 (7.7%) multi-organ. Increasing age was a risk factor for COVID-19 mortality, while vaccination within 180 days of COVID-19 diagnosis was protective in Cox proportional hazard models with hazard ratio 1.04 (95% CI: 1.01–1.06) and 0.31 (0.11–0.90), respectively). These findings were confirmed in the propensity score matched cohort between vaccinated and unvaccinated patients.
Conclusions
This investigation found COVID-19 mortality may be significantly reduced among immunosuppressed SOT recipients within 6 months following vaccination. These findings can inform vaccination policies targeting immunosuppressed populations worldwide.
Introduction
Solid organ transplant (SOT) recipients are at increased risk for morbidity and mortality from infectious diseases, including COVID-19, due to their immunosuppressed status and significant comorbidities [1,2]. At the end of 2020, the BNT162b2 (Pfizer-BioNTech) and the mRNA-1273 (Moderna) vaccines became available for high-risk populations, including transplant patients, and transplant centers across the United States instituted vaccination programs. However, recent studies have demonstrated poor humoral and cellular responses among vaccinated SOT recipients compared to patients on transplant waitlists and the general population, prompting concern for poor clinical outcomes of SOT recipients exposed to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus post-vaccination [3–20]. This investigation evaluated mortality among SOT recipients diagnosed with COVID-19 before and after implementation of an intensive vaccination program at a large, urban transplant center in Houston, Texas, USA.
Methods
The study population consisted of all consecutive SOT recipients diagnosed with COVID-19 between March 1, 2020, and October 1, 2021 at Houston Methodist, Houston Texas, USA. SARS-CoV-2 testing was performed as part of the routine screening on SOT recipients. Patients were followed up for outcomes up to December 1, 2021. The Houston Methodist J.C. Walter Jr. Transplant Center is located within the large urban, Texas Medical Center and maintains an active transplant and follow-up program including liver, heart, lung, pancreas, kidney, and multi-organ transplants. COVID-19 mRNA vaccines became available to high-risk populations in the study setting in December 2020, and vaccination programs utilizing the NT162b2 (Pfizer-BioNTech) or the mRNA-1273 (Moderna) vaccines for transplant patients began in January 2021. Demographic and clinical data were retrieved from the Houston Methodist COVID-19 Surveillance and Outcomes Registry (CURATOR), a COVID-19 specific electronic health records (EHR) data mining, surveillance and collection project [21]. Vaccination records and medication data were abstracted directly from the EHR. The Charlson Comorbidity Index was calculated from medical history components as a measure of overall comorbidity burden [22]. SOT recipients were included if they received a positive diagnostic result from a SARS-CoV-2 RNA polymerase chain reaction (PCR) assay or a viral antigen assay at Houston Methodist while undergoing immunosuppressive treatment.
Patients were considered ‘fully vaccinated’ in this study if they had received the second dose of either the NT162b2 or the mRNA-1273 vaccines at least 14 days prior to their first positive SARS-CoV-2 diagnostic test, according to CDC definitions [23]. Patients who tested positive for COVID-19 before the completion of the vaccination course were considered ‘unvaccinated’. Demographic and clinical data were reported as frequencies and proportions for categorical variables and as median and interquartile range (IQR) for continuous variables. Differences in covariates across vaccination status groups were evaluated using the chi-square or Fisher’s exact tests for categorical variables and Kruskal Wallis test for continuous variables, as appropriate. Timepoints for primary and secondary outcome analyses were determined from the distribution of time-to-event data. Cox proportional hazards modeling was performed to determine the characteristics associated with COVID-19 mortality within 60 days. Multivariable logistic regression modeling was utilized to determine the characteristics associated with an ICU admission within 30 days. Variables for the multivariable models were selected on the basis of potential clinical relevance and using the Bayesian Information Criterion. Only baseline characteristics were assessed in regression analyses, as eligibility for specific COVID-19 treatments, including remdesivir and monoclonal antibodies depended on additional factors, such as disease severity upon presentation and time from symptom onset [24,25]. Given the vaccine effectiveness drop after 6 months [26,27], we classified the vaccination status as follow: vaccination status was presented as ‘vaccinated <180 days before COVID-19 diagnosis’, ‘vaccinated 180+ days before COVID-19 diagnosis’, ‘unvaccinated, diagnosed with COVID-19 in 2020’, or ‘unvaccinated, diagnosed with COVID-19 in 2021.’ Unvaccinated patients were categorized by whether they were diagnosed with COVID-19 in 2020 or 2021 as a proxy for advances in COVID-19 treatments which could have influenced survivorship. We conducted a non-replace propensity score (PS) matching (ratio 1:1, caliper 1) between patients with and without vaccination. The matching criteria included age, race, Charlson Comorbidity Index (CCI), year of transplantation, COVID-19 treatments administered at diagnostic encounter (remdesivir, monoclonal antibodies, azithromycin, methylprednisolone, ribavirin, tocilizumab, dexamethasone), immunosuppressant treatments at COVID-19 diagnosis (antithymocyte globulin, tacrolimus, cyclosporine, mycophenolate, azathioprine, sirolimus, everolimus, belatacept, prednisone). Balance of the matching criteria between groups was evaluated using standardized bias percent. Multivariable analysis was also conducted on the PS cohort for mortality with 60 days and ICU admission within 30 days. All analyses were performed on Stata MP version 17.0 (StataCorp LLC, College Station, TX, USA). This retrospective registry-based study was approved by the Houston Methodist institutional review board (PRO00025320) and granted a waiver of informed consent.
Results
In total, 646 SOT recipients were diagnosed with COVID-19 at Houston Methodist from March 1, 2020 to October 1, 2021. SARS-CoV-2 -positive SOT recipients had a median age of 58 years (47–66 IQR); 357 (55%) were male, 251 (39%) were non-Hispanic White, 161 (25%) non-Hispanic Black, 30 (5%) non-Hispanic Asian, 193 (30%) Hispanic, and 11 (2%) classified as other non-Hispanic race (Table 1). This cohort of SOT recipients had significant comorbidities prior to COVID-19 diagnosis; median score on the Charlson Comorbidity Index was 8 (IQR 5–11), and almost all patients had history of renal disease or diabetes. In total, 315 (57%) SOT patients diagnosed with COVID-19 were kidney recipients, 92 (16%) liver recipients, 59 (11%) lung recipients, 56 (10%) heart recipients, and 36 (6%) multi-organ recipients. Median time from the latest transplant to date of first positive SARS-CoV-2 test was 4.6 years (1.4–9.2 IQR). Within the population of patients undergoing immunosuppressant therapy, 574 (89%) were receiving tacrolimus, 539 (83%) were receiving mycophenolate, and 561 (87%) were receiving prednisone at time of their COVID-19 diagnosis.
Table 1. Demographics and clinical characteristics of solid organ transplant recipients diagnosed with COVID-19 by vaccination status.
| Total | Not vaccinated | Vaccinated | ||
|---|---|---|---|---|
| Characteristics | (N = 646) | (n = 505) | (n = 141) | p-value |
| Demographics | ||||
| Age at encounter (years), median (IQR) | 58 (47–66) | 57 (46–65) | 61 (52–68) | 0.003 |
| Gender | 0.77 | |||
| Female | 289 (44.7%) | 224 (44.4%) | 65 (46.1%) | |
| Male | 357 (55.3%) | 281 (55.6%) | 76 (53.9%) | |
| Race/Ethnicity | 0.66 | |||
| Non-Hispanic White | 251 (38.9%) | 195 (38.6%) | 56 (39.7%) | |
| Non-Hispanic Black | 161 (24.9%) | 121 (24.0%) | 40 (28.4%) | |
| Non-Hispanic Asian | 30 (4.6%) | 21 (4.2%) | 9 (6.4%) | |
| Non-Hispanic Hawaiian/Pacific | 2 (0.3%) | 2 (0.4%) | 0 (0.0%) | |
| Non-Hispanic Native American | 4 (0.6%) | 4 (0.8%) | 0 (0.0%) | |
| Non-Hispanic Other Race | 2 (0.3%) | 2 (0.4%) | 0 (0.0%) | |
| Hispanic or Latino | 193 (29.9%) | 157 (31.1%) | 36 (25.5%) | |
| Unknown | 3 (0.5%) | 3 (0.6%) | 0 (0.0%) | |
| Clinical characteristics | ||||
| Body mass index, median (IQR) | 28.0 (24.3–32.4) | 28.0 (24.3–32.2) | 27.8 (24.4–33.0) | 0.51 |
| Charlson Comorbidity Index Score, median (IQR) | 8 (5–11) | 8 (5–11) | 8 (6–11) | 0.046 |
| Medical history | ||||
| Chronic obstructive pulmonary disease | 247 (38.2%) | 186 (36.8%) | 61 (43.3%) | 0.17 |
| Tuberculosis | 22 (3.3%) | 15 (3.0%) | 7 (5.0%) | 0.29 |
| Dementia | 22 (3.4%) | 20 (4.0%) | 2 (1.4%) | 0.19 |
| Myocardial Infarction | 226 (35.0%) | 169 (33.5%) | 57 (40.4%) | 0.13 |
| Peripheral vascular disease | 329 (50.9%) | 232 (45.9%) | 69 (48.9%) | 0.57 |
| Congestive heart failure | 301 (46.6%) | 255 (50.5%) | 74 (52.5%) | 0.7 |
| Cerebrovascular disease | 240 (37.2%) | 186 (36.8%) | 54 (38.3%) | 0.77 |
| Diabetes | 466 (72.1%) | 363 (71.9%) | 103 (73.0%) | 0.83 |
| Peptic ulcer disease | 58 (9.0%) | 42 (8.3%) | 16 (11.3%) | 0.32 |
| Liver disease | 269 (41.6%) | 209 (41.4%) | 60 (42.6%) | 0.85 |
| Renal disease | 620 (96.0%) | 485 (96.0%) | 135 (95.7%) | 0.81 |
| Hemiplegia | 20 (3.1%) | 19 (3.8%) | 1 (0.7%) | 0.09 |
| Cancer | 123 (19.0%) | 89 (17.6%) | 34 (24.1%) | 0.09 |
| HIV/AIDS | 3 (0.5%) | 2 (0.4%) | 1 (0.7%) | 0.52 |
| Transplant characteristics | ||||
| Transplanted organ | 0.62 | |||
| Multi-organ | 19 (2.9%) | 13 (2.6%) | 6 (4.3%) | |
| Heart | 63 (9.8%) | 53 (10.5%) | 10 (7.1%) | |
| Kidney | 355 (55.0%) | 274 (54.3%) | 81 (57.4%) | |
| Liver | 108 (16.7%) | 86 (17.0%) | 22 (15.6%) | |
| Lung | 70 (10.8%) | 53 (10.5%) | 17 (12.1%) | |
| Kidney/Pancreas | 31 (4.8%) | 26 (5.1%) | 5 (3.5%) | |
| Year of most recent transplant | 0.03 | |||
| <2015 | 254 (39.3%) | 200 (39.6%) | 54 (38.3%) | |
| 2015 | 47 (7.3%) | 38 (7.5%) | 9 (6.4%) | |
| 2016 | 43 (6.7%) | 34 (6.7%) | 9 (6.4%) | |
| 2017 | 56 (8.7%) | 44 (8.7%) | 12 (8.5%) | |
| 2018 | 49 (7.6%) | 41 (8.1%) | 8 (5.7%) | |
| 2019 | 87 (13.5%) | 76 (15.0%) | 11 (7.8%) | |
| 2020 | 88 (13.6%) | 58 (11.5%) | 30 (21.3%) | |
| 2021 | 22 (3.4%) | 14 (2.8%) | 8 (5.7%) | |
| Time from transplant to COVID-19 diagnosis (years), median IQR | 4.6 (1.4–9.2) | 4.6 (1.4–9.2) | 4.8 (1.3–9.5) | 0.62 |
| Immunosuppressant treatments at COVID-19 diagnosis | ||||
| Antithymocyte globulin | 21 (3.3%) | 20 (4.0%) | 1 (0.7%) | 0.06 |
| Tacrolimus | 574 (88.9%) | 451 (89.3%) | 123 (87.2%) | 0.49 |
| Cyclosporine | 39 (6.0%) | 30 (5.9%) | 9 (6.4%) | 0.85 |
| Mycophenolate | 539 (83.4%) | 422 (83.6%) | 117 (83.0%) | 0.87 |
| Azathioprine | 14 (2.2%) | 13 (2.6%) | 1 (0.7%) | 0.32 |
| Sirolimus | 61 (9.4%) | 49 (9.7%) | 12 (8.5%) | 0.67 |
| Everolimus | 14 (2.2%) | 9 (1.8%) | 5 (3.5%) | 0.20 |
| Belatacept | 14 (2.2%) | 12 (2.4%) | 2 (1.4%) | 0.75 |
| Prednisone | 561 (86.8%) | 434 (85.9%) | 127 (90.1%) | 0.20 |
| COVID-19 characteristics | ||||
| Date of COVID-19 diagnosis (quarter) | <0.001 | |||
| Q1 2020 | 4 (0.6%) | 4 (0.8%) | 0 (0.0%) | |
| Q2 2020 | 68 (10.5%) | 68 (13.5%) | 0 (0.0%) | |
| Q3 2020 | 106 (16.4%) | 106 (21.0%) | 0 (0.0%) | |
| Q4 2020 | 122 (18.9%) | 122 (24.2%) | 0 (0.0%) | |
| Q1 2021 | 138 (21.4%) | 131 (25.9%) | 7 (5.0%) | |
| Q2 2021 | 44 (6.8%) | 22 (4.4%) | 22 (15.6%) | |
| Q3 2021 | 164 (25.4%) | 52 (10.3%) | 112 (79.4%) | |
| COVID-19 treatments administered at diagnostic encounter | ||||
| Azithromycin | 154 (23.8%) | 129 (25.5%) | 25 (17.7%) | 0.05 |
| Methylprednisolone | 130 (20.1%) | 102 (20.2%) | 28 (19.9%) | 0.93 |
| Ribavirin | 6 (0.9%) | 6 (1.2%) | 0 (0.0%) | 0.35 |
| Tocilizumab | 38 (5.9%) | 37 (7.3%) | 1 (0.7%) | 0.002 |
| Dexamethasone | 225 (34.8%) | 176 (34.9%) | 49 (34.8%) | 0.98 |
| Remdesivir | 231 (35.8%) | 169 (33.5%) | 62 (44.0%) | 0.02 |
| Monoclonal antibodies | 136 (21.1%) | 76 (15.0%) | 60 (42.6%) | <0.001 |
| Clinical outcomes | ||||
| Total mortality | 0.01 | |||
| Alive at study completion | 557 (86.2%) | 426 (84.4%) | 131 (92.9%) | |
| Expired | 89 (13.8%) | 79 (15.6%) | 10 (7.1%) | |
| Cause of death (n = 89) | 0.85 | |||
| Graft rejection/failure | 6 (6.7%) | 6 (8%) | 0 (0%) | |
| Cardiac arrest | 3 (3.4%) | 3 (4%) | 0 (0%) | |
| Respiratory failure | 3 (3.4%) | 3 (4%) | 0 (0%) | |
| Multi-organ failure | 1 (1.1%) | 1 (1%) | 0 (0%) | |
| Sepsis | 2 (2.3%) | 2 (3%) | 0 (0%) | |
| COVID-19 | 70 (78.7%) | 61 (77%) | 9 (90.0%) | |
| Myocardial infarction | 1 (1.2%) | 1 (1%) | 0 (0%) | |
| Other | 3 (3.4%) | 2 (3%) | 1 (10.0%) | |
| Patient status 30 days post COVID-19 diagnosis | ||||
| Ever hospitalized | 473 (73.2%) | 384 (76.0%) | 89 (63.1%) | 0.004 |
| Ever admitted to ICU | 136 (21.1%) | 117 (23.2%) | 19 (13.5%) | 0.01 |
| Expired | 56 (8.7%) | 47 (9.3%) | 9 (6.4%) | 0.31 |
| Cause of death within 30 days of diagnosis (n = 56) | 0.30 | |||
| COVID-19 | 54 (96%) | 46 (98%) | 8 (89%) | |
| Other | 2 (4%) | 1 (2%) | 1 (11%) |
Values are in number (%) unless otherwise specified; IQR: Interquartile range; ICU: Intensive care unit; Differences between exposure groups were compared using the chi-square or Fisher’s exact tests for categorical variables and Kruskal Wallis test for continuous variables.
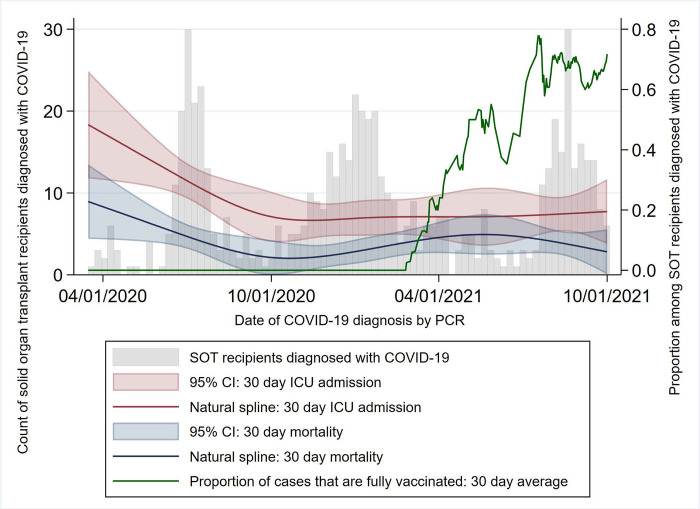
COVID-19 incidence among SOT recipients followed local epidemiologic trends, displaying four distinct peaks in April 2020, June 2020, January 2021, and August 2021 [28] (Fig 1). Following the initial peak, during which testing and treatment policies varied widely [21,29], 30 day mortality and 30 day ICU admission rates remained relatively stable, even as the proportion of patients diagnosed with COVID-19 after completing a vaccination course increased. In total, 300 (46%) SOT recipients were diagnosed with COVID-19 in 2020, before vaccinations were available, while 205 (32%) were diagnosed in 2021, but were not fully vaccinated at time of diagnosis. Within the latter category, 31/205 (15%) had received at least one vaccine dose but were not considered fully vaccinated under the CDC definition. Among the remaining patients, 90 (14%) completed their vaccination course within the 179 days prior to their diagnosis, while 51 (8%) were vaccinated 180 days or more before their diagnosis; median time from vaccine course completion to COVID-19 diagnosis was 160 days (IQR 108–190 days). The study cohort did not contain any transplant patients received the viral vector JNJ-78436735 (Johnson & Johnson) vaccine. Among all study patients, 154 (24%) received azithromycin, 130 (20%) received methylprednisolone, and 225 (35%) received dexamethasone within their diagnostic encounter. In addition, 136 (21%) patients received monoclonal antibody treatment for COVID-19. Of 473 patients hospitalized within 30 days of COVID-19 diagnosis, 231 (49%) received remdesivir for COVID-19 treatment.
Fig 1. COVID-19 in solid organ transplant recipients over time.
SOT: Solid organ transplant; ICU: Intensive care unit; CI: Confidence interval.
In total, of 646 SOT recipients under immunosuppressant regimens who were diagnosed with COVID-19 between March 1, 2020 and October 1, 2021, 99 (15.3%) expired before December 1, 2021, and 70 (79%) of those had COVID-19 listed as the primary cause of death with 66/70 (94.3%) patients died within 60 days of diagnosis. Median survival time for all expired patients was 28 days from COVID-19 diagnosis (IQR 15–63 days, range 1–585 days), while median survival time for patients who died from COVID-19 was 21 days (IQR 13–29 days, range 1–82 days) (Fig 1). The primary outcome of COVID-19 mortality within 60 days of diagnosis was used in survival analyses. Of the 66 patients who died from COVID-19 within 60 days of diagnosis, 57 (86%) were unvaccinated, 4 (6%) had been vaccinated less than 180 days before being diagnosed, and 5 (8%) had been vaccinated 180 days or more before being diagnosed. In multivariable Cox proportional hazard models, increasing age was a risk factor for 60 day COVID-19 mortality with hazard ratio (HR): 1.04 (5% CI: 1.01–1.16); p-value: 0.001, while vaccination within 180 days of diagnosis was protective, compared to unvaccinated patients diagnosed in 2021, HR: 0.31 (95% CI 0.11–0.90); p-value: 0.03. Notably, vaccination at 180 days or longer was not protective against COVID-19 mortality, HR 0.70 (95% CI 0.26–1.85); p-value: 0.47; furthermore, mortality risk did not vary significantly between SOT recipients diagnosed in 2020 and unvaccinated SOT recipients diagnosed in 2021 (Table 2).
Table 2. 60-day COVID-19 mortality among solid organ transplant recipients.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Cox Proportional Hazard model | (N = 646) | (N = 646) | ||
| Event: COVID-19 mortality, n = 66 | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age at encounter (years) | 1.03 (1.01, 1.05) | 0.01 | 1.04 (1.01, 1.06) | 0.001 |
| Gender | ||||
| Female | (reference) | (reference) | ||
| Male | 0.85 (0.53, 1.38) | 0.52 | 0.79 (0.49, 1.29) | 0.36 |
| Race/Ethnicity | ||||
| NH White | (reference) | |||
| NH Black | 1.31 (0.72, 2.37) | 0.37 | ||
| NH Asian | 0.70 (0.16, 2.9) | 0.62 | ||
| NH Other Race | 0.98 (0.13, 7.26) | 0.99 | ||
| Hispanic or Latino | 1.02 (0.56, 1.86) | 0.96 | ||
| Body Mass Index | 1.02 (0.99, 1.06) | 0.22 | ||
| Charlson Comorbidity Index Score | 1.04 (0.99, 1.10) | 0.13 | ||
| Transplanted organ | ||||
| Multi-organ | 0.96 (0.23, 3.97) | 0.95 | 1.04 (0.25, 4.37) | 0.96 |
| Heart | 0.58 (0.21, 1.62) | 0.29 | 0.46 (0.16, 1.30) | 0.14 |
| Kidney | (reference) | (reference) | ||
| Liver | 0.84 (0.42, 1.67) | 0.61 | 0.70 (0.34, 1.40) | 0.31 |
| Lung | 1.04 (0.48, 2.22) | 0.92 | 0.90 (0.42, 1.93) | 0.78 |
| Kidney/Pancreas | 0.85 (0.26, 2.75) | 0.79 | 1.09 (0.33, 3.58) | 0.89 |
| Time from transplant to COVID-19 diagnosis (years) | 1.02 (0.98, 1.06) | 0.27 | ||
| Vaccination status | ||||
| Unvaccinated, diagnosed in 2020 | 0.93 (0.55, 1.57) | 0.78 | 0.92 (0.54, 1.55) | 0.75 |
| Unvaccinated, diagnosed in 2021 | (reference) | (reference) | ||
| Diagnosed <180 days after vaccination | 0.36 (0.13, 1.05) | 0.06 | 0.31 (0.11, 0.90) | 0.03 |
| Diagnosed 180+ days after vaccination | 0.84 (0.32, 2.20) | 0.72 | 0.70 (0.26, 1.85) | 0.47 |
Model notes: Includes all SOT recipients receiving immunosuppressant treatments at time of COVID-19 diagnosis. Outcome: Mortality from COVID-19 within 60 days of COVID-19 diagnosis; HR, hazard ratio; CI, confidence interval.
Of 646 SOT recipients, 136 (21%) were admitted to the ICU within 30 days of COVID-19 diagnosis; 117/136 (86%) of patients admitted to the ICU were unvaccinated. In multivariable logistic regression analysis, an increasing Charlson Comorbidity Index score was a risk factor for 30-day ICU admission, odds ratio (OR): 1.09 (95% CI 1.04–1.15); p-value: <0.001, while vaccination within 180 days of diagnosis was again protective, compared to unvaccinated patients diagnosed in 2021, OR: 0.45 (0.21–0.95); p-value: 0.04 (Table 3).
Table 3. Risk of ICU admission among solid organ transplant recipients diagnosed with COVID-19.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Logistic regression model | (N = 646) | (N = 626) | ||
| Event: ICU admission, n = 136 | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age at encounter (years) | 1.01 (1.00, 1.03) | 0.051 | -- | -- |
| Gender | ||||
| Female | (reference) | -- | -- | |
| Male | 1.11 (0.76, 1.63) | 0.58 | -- | -- |
| Race/Ethnicity | ||||
| NH White | (reference) | -- | -- | |
| NH Black | 1.13 (0.70, 1.81) | 0.62 | -- | -- |
| NH Asian | 0.41 (0.12, 1.39) | 0.15 | -- | -- |
| NH Other Race | 0.36 (0.05, 2.91) | 0.34 | -- | -- |
| Hispanic or Latino | 0.95 (0.60, 1.51) | 0.84 | -- | -- |
| Body Mass Index | 1.02 (0.99, 1.05) | 0.27 | 1.02 (0.99, 1.05) | 0.18 |
| Charlson Comorbidity Index Score | 1.08 (1.04, 1.13) | <0.001 | 1.09 (1.04, 1.15) | <0.001 |
| Transplanted organ | ||||
| Multi-organ | 1.38 (0.48, 3.95) | 0.55 | 1.34 (0.44, 4.10) | 0.61 |
| Heart | 0.73 (0.35, 1.50) | 0.39 | 0.53 (0.25, 1.12) | 0.1 |
| Kidney | (reference) | (reference) | ||
| Liver | 1.10 (0.66, 1.86) | 0.71 | 0.72 (0.40, 1.29) | 0.27 |
| Lung | 1.44 (0.80, 2.59) | 0.22 | 1.16 (0.62, 2.16) | 0.64 |
| Kidney/Pancreas | 0.74 (0.28, 2.00) | 0.56 | 0.76 (0.28, 2.10) | 0.6 |
| Time from transplant to COVID-19 diagnosis (years) | 1.00 (0.97, 1.04) | 0.85 | ||
| Vaccination status | ||||
| Unvaccinated, diagnosed in 2020 | 1.29 (0.84, 1.99) | 0.24 | 1.30 (0.84, 2.01) | 0.25 |
| Unvaccinated, diagnosed in 2021 | (reference) | (reference) | ||
| Diagnosed <180 days after vaccination | 0.49 (0.23, 1.02) | 0.06 | 0.45 (0.21, 0.95) | 0.04 |
| Diagnosed 180+ days after vaccination | 0.83 (0.38, 1.84) | 0.65 | 0.68 (0.30, 1.53) | 0.35 |
Model notes: Includes all SOT recipients receiving immunosuppressant treatments at time of COVID-19 diagnosis. Outcome: ICU admission within 30 days of COVID-19 diagnosis; OR, odds ratio; CI: Confidence interval; ICU: Intensive care unit.
Patient characteristics in the PS matched cohort was present in Table 4, which showed all evaluated criteria were balanced between patients with and without vaccination.
Table 4. Demographics and clinical characteristics of solid organ transplant recipients diagnosed with COVID-19 by vaccination status, propensity score matched cohort.
| Total | Not vaccinated | Vaccinated | Standardized | p-value | |
|---|---|---|---|---|---|
| Characteristics | N = 282 | N = 141 | N = 141 | bias % | |
| Demographics | |||||
| Age at encounter (years), median (IQR) | 60.0 (52.0, 67.0) | 59.0 (51.0, 66.0) | 61.0 (52.0, 68.0) | 7.5 | 0.53 |
| Gender | |||||
| Female | 127 (45.0) | 62 (44.0) | 65 (46.1) | 4.3 | 0.72 |
| Male | 155 (55.0) | 79 (56.0) | 76 (53.9) | -4.3 | 0.72 |
| Race/Ethnicity | |||||
| Non-Hispanic White | 121 (42.9) | 65 (46.1) | 56 (39.7) | -12.9 | 0.28 |
| Non-Hispanic Black | 69 (24.5) | 29 (20.6) | 40 (28.4) | 18.2 | 0.13 |
| Non-Hispanic Asian | 15 (5.3) | 6 (4.3) | 9 (6.4) | 9.5 | 0.43 |
| Hispanic or Latino | 77 (27.3) | 41 (29.1) | 36 (25.5) | -7.9 | 0.51 |
| Clinical characteristics | |||||
| Body mass index, median (IQR) | 28.3 (24.3, 32.8) | 28.3 (24.3, 32.5) | 27.8 (24.4, 33.0) | 0.65 | |
| Charlson Comorbidity Index Score, median (IQR) | 8.5 (6.0, 11.0) | 9.0 (6.0, 11.0) | 8.0 (6.0, 11.0) | 0.7 | 0.95 |
| Medical history | |||||
| Chronic obstructive pulmonary disease | 123 (43.6) | 62 (44.0) | 61 (43.3) | -1.4 | 0.91 |
| Tuberculosis | 4 (1.4) | 2 (1.4) | 2 (1.4) | 0.0 | 1.00 |
| Dementia | 103 (36.5) | 46 (32.6) | 57 (40.4) | 16.2 | 0.18 |
| Myocardial Infarction | 137 (48.6) | 68 (48.2) | 69 (48.9) | 1.4 | 0.91 |
| Peripheral vascular disease | 155 (55.0) | 81 (57.4) | 74 (52.5) | -10.0 | 0.40 |
| Congestive heart failure | 118 (41.8) | 64 (45.4) | 54 (38.3) | -14.4 | 0.23 |
| Cerebrovascular disease | 29 (10.3) | 13 (9.2) | 16 (11.3) | 7.0 | 0.56 |
| Diabetes | 118 (41.8) | 58 (41.1) | 60 (42.6) | 2.9 | 0.81 |
| Peptic ulcer disease | 211 (74.8) | 108 (76.6) | 103 (73.0) | -8.1 | 0.49 |
| Liver disease | 6 (2.1) | 5 (3.5) | 1 (0.7) | -19.7 | 0.10 |
| Renal disease | 272 (96.5) | 137 (97.2) | 135 (95.7) | -7.6 | 0.52 |
| Hemiplegia | 65 (23.0) | 31 (22.0) | 34 (24.1) | 5.0 | 0.67 |
| Cancer | 26 (9.2) | 11 (7.8) | 15 (10.6) | 9.8 | 0.41 |
| HIV/AIDS | 2 (0.7) | 1 (0.7) | 1 (0.7) | 0.0 | 1.00 |
| Transplant characteristics | |||||
| Transplanted organ | |||||
| Multi-organ | 9 (3.2) | 3 (2.1) | 6 (4.3) | 12.1 | 0.31 |
| Heart | 26 (9.2) | 16 (11.3) | 10 (7.1) | -14.7 | 0.22 |
| Kidney | 147 (52.1) | 66 (46.8) | 81 (57.4) | 21.3 | 0.07 |
| Liver | 51 (18.1) | 29 (20.6) | 22 (15.6) | -12.9 | 0.28 |
| Lung | 37 (13.1) | 20 (14.2) | 17 (12.1) | -6.3 | 0.60 |
| Kidney/Pancreas | 12 (4.3) | 7 (5.0) | 5 (3.5) | -7.0 | 0.56 |
| Year of most recent transplant | |||||
| <2015 | 95 (33.7) | 41 (29.1) | 54 (38.3) | 19.5 | 0.10 |
| 2015 | 24 (8.5) | 15 (10.6) | 9 (6.4) | -15.2 | 0.20 |
| 2016 | 19 (6.7) | 10 (7.1) | 9 (6.4) | -2.8 | 0.81 |
| 2017 | 27 (9.6) | 15 (10.6) | 12 (8.5) | -7.2 | 0.55 |
| 2018 | 20 (7.1) | 12 (8.5) | 8 (5.7) | -11.0 | 0.36 |
| 2019 | 35 (12.4) | 24 (17.0) | 11 (7.8) | -28.1 | 0.02 |
| 2020 | 49 (17.4) | 19 (13.5) | 30 (21.3) | 20.6 | 0.08 |
| 2021 | 13 (4.6) | 5 (3.5) | 8 (5.7) | 10.1 | 0.40 |
| Time from transplant to COVID-19 diagnosis (years), median IQR | 4.4 (1.4, 8.5) | 3.8 (1.4, 6.9) | 4.8 (1.3, 9.5) | 12.6 | 0.29 |
| Immunosuppressant treatments at COVID-19 diagnosis | |||||
| Antithymocyte globulin | 2 (0.7) | 1 (0.7) | 1 (0.7) | 0.0 | 1.00 |
| Tacrolimus | 250 (88.7) | 127 (90.1) | 123 (87.2) | -8.9 | 0.45 |
| Cyclosporine | 14 (5.0) | 5 (3.5) | 9 (6.4) | 13.0 | 0.27 |
| Mycophenolate | 238 (84.4) | 121 (85.8) | 117 (83.0) | -7.8 | 0.51 |
| Azathioprine | 1 (0.4) | 0 (0.0) | 1 (0.7) | 11.9 | 0.32 |
| Sirolimus | 25 (8.9) | 13 (9.2) | 12 (8.5) | -2.5 | 0.84 |
| Everolimus | 9 (3.2) | 4 (2.8) | 5 (3.5) | 4.0 | 0.74 |
| Belatacept | 4 (1.4) | 2 (1.4) | 2 (1.4) | 0.0 | 1.00 |
| Prednisone | 254 (90.1) | 127 (90.1) | 127 (90.1) | 0.0 | 1.00 |
| COVID-19 treatments administered at diagnostic encounter | |||||
| Azithromycin | 55 (19.5) | 30 (21.3) | 25 (17.7) | -8.9 | 0.45 |
| Methylprednisolone | 63 (22.3) | 35 (24.8) | 28 (19.9) | -11.9 | 0.32 |
| Ribavirin | 0 (0.0) | 0 (0.0) | 0 (0.0) | . | . |
| Tocilizumab | 3 (1.1) | 2 (1.4) | 1 (0.7) | -6.9 | 0.56 |
| Dexamethasone | 98 (34.8) | 49 (34.8) | 49 (34.8) | 0.0 | 1.00 |
| Remdesivir | 127 (45.0) | 65 (46.1) | 62 (44.0) | -4.3 | 0.72 |
| Monoclonal antibodies | 122 (43.3) | 62 (44.0) | 60 (42.6) | -2.9 | 0.81 |
IQR: Interquartile range; ICU: Intensive care unit; Differences between exposure groups compared using chi-square or Fisher’s exact tests for categorical variables and Kruskal Wallis test for continuous variables.
The Cox regression model run on the PS matched cohort confirmed the association between the vaccination and lower mortality within 60 days with a multivariable HR of 0.28 (95% CI 0.08, 0.94), p = 0.04 (Table 5). The multivariable logistic regression model run on the PS matched cohort also found patients who received vaccination with 180 days from diagnosis had lower odds of ICU admission, OR 0.21 (95% CI 0.08, 0.51), p = 0.001 (Table 6).
Table 5. 60-day COVID-19 mortality among solid organ transplant recipients, in propensity score matched cohort (N = 282).
| Multivariable | ||
|---|---|---|
| Characteristics | HR (95% CI) | p-value |
| Age at encounter (years) | 1.04 (1.00, 1.08) | 0.06 |
| Vaccination status | ||
| Unvaccinated, diagnosed in 2020 | 0.55 (0.20, 1.51) | 0.24 |
| Unvaccinated, diagnosed in 2021 | REF | |
| Diagnosed <180 days after vaccination | 0.28 (0.08, 0.94) | 0.04 |
| Diagnosed 180+ days after vaccination | 0.77 (0.25, 2.37) | 0.65 |
| Monoclonal antibodies | 0.05 (0.01, 0.36) | 0.003 |
HR, hazard ratio; CI, confidence interval; REF, reference group.
Table 6. Risk of ICU admission among solid organ transplant recipients diagnosed with COVID-19, in the propensity score matched cohort (N = 282).
| Multivariable | ||
|---|---|---|
| Characteristics | OR (95% CI) | p-value |
| Age at encounter (years) | 0.99 (0.96, 1.02) | 0.41 |
| Male gender | 1.30 (0.67, 2.50) | 0.44 |
| Charlson Comorbidity Index Score | 1.16 (1.06, 1.28) | 0.002 |
| Transplanted organ | ||
| Multi-organ | 1.10 (0.17, 6.92) | 0.92 |
| Heart | 0.69 (0.22, 2.17) | 0.53 |
| Kidney | REF | |
| Liver | 1.02 (0.41, 2.51) | 0.97 |
| Lung | 1.63 (0.58, 4.55) | 0.35 |
| Kidney/Pancreas | 1.50 (0.30, 7.90) | 0.65 |
| Vaccination status | ||
| Unvaccinated, diagnosed in 2020 | 0.58 (0.25, 1.36) | 0.21 |
| Unvaccinated, diagnosed in 2021 | REF | |
| Diagnosed <180 days after vaccination | 0.21 (0.08, 0.51) | 0.001 |
| Diagnosed 180+ days after vaccination | 0.40 (0.15, 1.06) | 0.07 |
| Monoclonal antibodies | 0.19 (0.08, 0.41) | <0.001 |
OR, odds ratio; CI, confidence interval; REF, reference group.
Discussion
Our findings add to the growing body of literature demonstrating that, in spite of chronic immunosuppression and ostensibly a poor humoral response to the mRNA vaccines, mortality may be reduced among fully vaccinated solid organ transplant recipients who are exposed to COVID-19, compared to partially vaccinated or unvaccinated patients [30–32]. The presence of neutralizing antibodies could plausibly contribute to the observed reduction in COVID-19 case fatality among fully vaccinated SOT patients. However, new research indicates the production of neutralizing antibodies may also be hindered by the immunosuppressive drugs used in SOT recipients [33,34]. Therefore, future research is needed to identify additional mechanisms, such as a potentially protective role of cellular (T-cell) immunity, to explain the protective effect of vaccination without accompanying immunogenicity [35]. Additionally, further investigation should determine the effect of novel SARS-CoV-2 variants on disease dynamics within immunosuppressed populations. We also demonstrated that vaccination may offer waning protection from poor COVID-19 outcomes over time in an immunosuppressed population; SOT recipients in our cohort diagnosed with COVID-19 six months or more after being vaccinated were at similar risk for both COVID-19 mortality and ICU admission as patients who were never vaccinated. This study was restricted to patients without a known history of previous COVID-19 infection, and further research is needed to determine how vaccine immunogenicity may vary among immunosuppressed survivors of COVID-19.
Our study has several limitations. First, given this is a retrospective study, some clinical parameters were not collected in our dataset. For example, time from the first symptoms to diagnosis of COVID-19 was not available which may affect the outcome as we could not determine if the vaccinated patients were more likely to seek medical care earlier than unvaccinated patients. However, the analysis on the PS matched cohort confirmed that vaccination within 180 days of diagnosis was associated with a lower mortality within 60 days of diagnosis or ICU admission, independently with the treatment of remdesivir or monoclonal antibodies. Second, our data were obtained from one hospital system, which might not be generalized to other populations. Third, data of patients who had at least three doses of vaccines were not available in our patients for the time being. Of note, while patients in our study were considered as “fully vaccinated” with 2 vaccine doses as per the CDC definition, SOT recipients ages 5 years and older are recommended by the American Society of Transplantation to receive an initial three-dose series of mRNA vaccine followed by one or two booster doses if ages 12 or older [36].” Fourth, although we tried our best to rule out patients with a known history of previous COVID-19 infection, we may have missed some previous infections given lack of the confirmation of anti-N SARS-CoV-2 antibodies. Our analysis may also underestimate the patient outcome as we could not rule out completely the possibility that the patient sought further care at a different institution after their initial encounter at the Houston Methodist Hospital System. Finally, our findings may need to be considered with a gap between the data collected during the pandemic period at a single center and the current COVID-19 situation, especially with the fast-pace of evolving new SARS-CoV-2 variants and the change in the vaccine recommendations for SOT recipients from 2 doses to a primary 3-dose course followed by boosters.
Despite the limitations, our study has notable strengths. Our study is strengthened by a robust sample size of SOT recipients with COVID-19, a diverse, heterogeneous population, precise EHR-derived clinical information, and significant longitudinal follow-up of COVID-19 patients. While overall COVID-19 case fatality rates have decreased over time following the introduction of more effective treatment strategies [10], among our population of immunosuppressed SOT recipients, COVID-19 mortality and ICU admission rates did not vary significantly after the initial peak in April 2020. This investigation was a single-center, registry-based chart-review, so while patients could have experienced un-observed or un-documented outcomes at other institutions, all SOT recipients routinely received SARS-CoV-2 testing for surveillance purposes during the study period, regardless of symptoms. The observed difference between the fully vaccinated and unvaccinated patients can inform clinical practice and warrants additional studies as large-scale vaccination efforts continue. Moreover, the observed loss of protection within months of vaccination lends crucial evidence to discussions of booster recommendations in immunologically at-risk populations. Future investigations may prioritize longitudinal follow-up of vaccinated patients to determine incidence of additional markers of morbidity and poor health outcomes over time. This study demonstrated an association between mRNA vaccination and reduced mortality in solid organ transplant recipients; these results can contribute to the development of comprehensive vaccination programs targeting high-risk, immunosuppressed populations.
Acknowledgments
The authors thank the Houston Methodist Center for Outcome Research’s CURATOR team for providing data using in this analysis.
Abbreviations
- SOT
sold organ transplant
- IQR
Interquartile range
- HR
hazard ratio
- OR
odds ratio
- CI
confidence interval
- ICU
Intensive care unit
Data Availability
Data cannot be shared publicly because of patient confidentiality concerns as imposed by the Houston Methodist Institutional Review Board. Access to de-identified data can be made to Sonya Hadrigan (smhadrigan@houstonmethodist.org) which will be evaluated on a case by case basis in line with institutional policies
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: A multi-center cohort study. Clin Infect Dis. 2021. Dec 6;73(11):e4090–e4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation. 2021;105(1):37–55. doi: 10.1097/TP.0000000000003523 [DOI] [PubMed] [Google Scholar]
- 3.Yi SG, Knight RJ, Graviss EA, et al. Kidney Transplant Recipients Rarely Show an Early Antibody Response Following the First COVID-19 Vaccine Administration. Transplantation. 2021;105(7):e72–e73. [DOI] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Barbur I, Chiang TP-Y, et al. SARS-CoV-2 Messenger RNA Vaccine Immunogenicity in Solid Organ Transplant Recipients With Prior COVID-19. Transplantation. 2021. Nov 1;105(11):e270–e271. doi: 10.1097/TP.0000000000003900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslam S, Adler E, Mekeel K, Little SJ. Clinical Effectiveness of COVID-19 Vaccination in Solid Organ Transplant Recipients. Transpl Infect Dis. 2021. Oct;23(5):e13705. doi: 10.1111/tid.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavarot N, Morel A, Leruez-Ville M, et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. American Journal of Transplantation. 2021. Dec;21(12):4043–4051. doi: 10.1111/ajt.16814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali NM, Alnazari N, Mehta SA, et al. Development of COVID-19 Infection in Transplant Recipients After SARS-CoV-2 Vaccination. Transplantation. 2021; Sep 1;105(9):e104–e106. doi: 10.1097/TP.0000000000003836 [DOI] [PubMed] [Google Scholar]
- 8.Anjan S, Natori Y, Fernandez Betances AA, et al. Breakthrough COVID-19 infections after mRNA vaccination in Solid Organ Transplant Recipients in Miami, Florida. Transplantation. 2021. Oct 1;105(10):e139–e141. doi: 10.1097/TP.0000000000003902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–2739. doi: 10.1111/ajt.16701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8): 2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallett AM, Greenberg RS, Boyarsky BJ, et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant. 2021. Dec;40(12):1579–1588. doi: 10.1016/j.healun.2021.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021. Dec;21(12):3971–3979. doi: 10.1111/ajt.16768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman TW, Meek B, Rijkers GT, van Kessel DA. Poor Serologic Response to Two Doses of an mRNA-Based SARS-CoV-2 Vaccine in Lung Transplant Recipients. Transplantation. 2021. Jun;72:101599. doi: 10.1016/j.trim.2022.101599 Epub 2022 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malinis M, Cohen E, Azar MM. Effectiveness of SARS-CoV-2 vaccination in fully vaccinated solid organ transplant recipients. Am J Transplant. 2021. Aug;21(8):2916–2918. doi: 10.1111/ajt.16713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzola A, Todesco E, Drouin S, et al. Poor Antibody Response after Two Doses of SARS-CoV-2 vaccine in Transplant Recipients. Clin Infect Dis. 2021. Mar 23;74(6):1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta RB, Silveira FP. COVID-19 after two doses of mRNA vaccines in kidney transplant recipients. Am J Transplant. 2021. Dec;21(12):4102–4104. doi: 10.1111/ajt.16778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021. Aug;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021. Dec;21(12):3990–4002. doi: 10.1111/ajt.16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schramm R, Costard-Jäckle A, Rivinius R, et al. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol. 2021. Aug;110(8):1142–1149. doi: 10.1007/s00392-021-01880-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanis A, Haddadin Z, Spieker AJ, Waqfi D, Rankin DA, Talj R, et al. Humoral and cellular immune responses to the SARS-CoV-2 BNT162b2 vaccine among a cohort of solid organ transplant recipients and healthy controls. Transpl Infect Dis. 2022. Feb;24(1):e13772. doi: 10.1111/tid.13772 Epub 2021 Dec 21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vahidy FS, Drews AL, Masud FN, et al. Characteristics and Outcomes of COVID-19 Patients During Initial Peak and Resurgence in the Houston Metropolitan Area. JAMA. 2020. Sep 8;324(10):998–1000. doi: 10.1001/jama.2020.15301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention. Data Definitions for COVID-19 Vaccinations in the United States. Updated 08/17/2022. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/reporting-vaccinations.html. Access on 09/18/2022. [Google Scholar]
- 24.FDA Approves First Treatment for COVID-19. US Food and Drug Administration. FDA News Release Web site. Published 2020. Updated October 22, 2020. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. Accessed November 1, 2021. [Google Scholar]
- 25.Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. US Food and Drug Administration. FDA News Release Web site. Published 2020. Updated November 09, 2020. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19. Accessed November 1, 2021. [Google Scholar]
- 26.Tartof SY, Slezak JM, Fischer H,et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021. Oct 16;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8 Epub 2021 Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright BJ, Tideman S, Diaz GA, French T, Parsons GT, Robicsek A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study. Lancet Respir Med. 2022. Jun;10(6):557–565. doi: 10.1016/S2213-2600(22)00042-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Texas COVID-19 Data. Texas Department of State Health Services Center for Health Statistics; 2021. Available at: https://dshs.texas.gov/coronavirus/AdditionalData.aspx. Accessed 3/1/2021. [Google Scholar]
- 29.Fauci AS, Lane HC, Redfield RR. Covid-19—Navigating the uncharted. N Engl J Med. 2020. Mar 26;382(13):1268–1269. doi: 10.1056/NEJMe2002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two Doses of SARS-CoV-2 Vaccines Reduce Risk of Death Due to COVID-19 in Solid Organ Transplant Recipients: Preliminary Outcomes From a UK Registry Linkage Analysis. Transplantation. 2021. Nov 1;105(11):e263–e264. doi: 10.1097/TP.0000000000003908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hod T, Ben-David A, Olmer L, et al. Humoral Response of Renal Transplant Recipients to the BNT162b2 SARS-CoV-2 mRNA Vaccine Using Both RBD IgG and Neutralizing Antibodies. Transplantation. 2021. Nov 1;105(11):e234–e243. doi: 10.1097/TP.0000000000003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinchera B, Spirito L, Ferreri L, et al. ; “Federico II” COVID-19 Team. SARS-CoV-2 in Kidney Transplant Patients: A Real-Life Experience. Front Med (Lausanne). 2022. Mar 28;9:864865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinchera B, Buonomo AR, Scotto R, et al. ; Federico II COVID team. Sotrovimab in Solid Organ Transplant Patients With Early, Mild/Moderate SARS-CoV-2 Infection: A Single-center Experience. Transplantation. 2022. Jul 1;106(7):e343–e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall VG, Ferreira VH, Ku T, et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. New England Journal of Medicine. 2021. Sep 23;385(13):1244–1246. doi: 10.1056/NEJMc2111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stock PG, Henrich TJ, Segev DL, Werbel WA. Interpreting and addressing suboptimal immune responses after COVID-19 vaccination in solid-organ transplant recipients. The Journal of Clinical Investigation. 2021. Jul 15;131(14);131(14):e151178. doi: 10.1172/JCI151178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Society of Transplantation. COVID-19 Vaccine FAQ Sheet (updated 5/4/2022). Available at https://www.myast.org/sites/default/files/2022.05.04%20AST%20Vaccine%20FAQ-CLEAN.pdf. Access on 09/18/2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared publicly because of patient confidentiality concerns as imposed by the Houston Methodist Institutional Review Board. Access to de-identified data can be made to Sonya Hadrigan (smhadrigan@houstonmethodist.org) which will be evaluated on a case by case basis in line with institutional policies