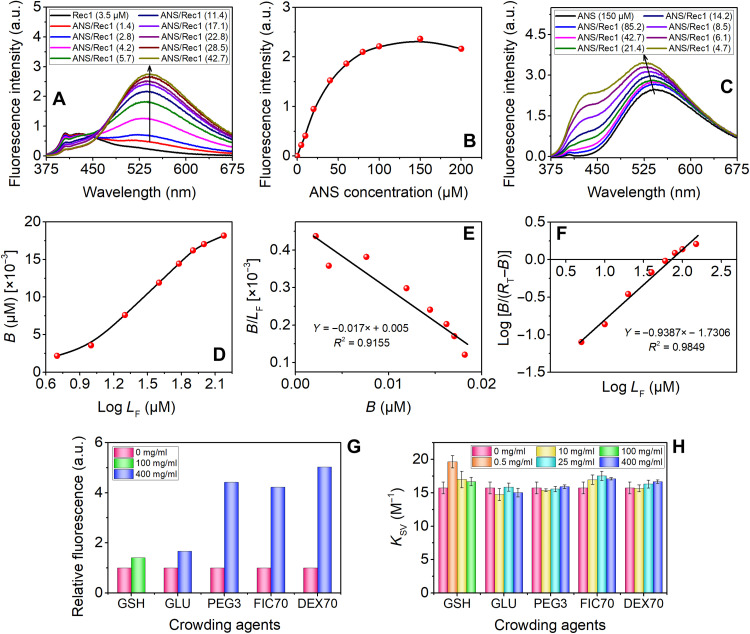
Fig. 6. Spectrofluorimetric assessment of the effect of crowders on conformational adjustment and surface hydrophobicity change using ANS as the extrinsic fluorescence probe and Tyr as the intrinsic fluorescence probe.
(A) Steady-state fluorescence emission spectra of pure Rec1-resilin and ANS/Rec1-resilin complexes at various molar ratios. (B) Change in λmax with increase in concentration of ANS in fixed Rec1-resilin. The maximum fluorescence intensity for the complex was observed at approximately ~42.7:1 ANS/Rec1-resilin ratio. (C) Fluorescence spectra of pure ANS and ANS/Rec1 complex with increasing Rec1 concentration. (D) Klotz plot: binding isotherm representing the number of moles of ANS bound per mole of protein. (E) Scatchard treatment of the ANS binding data. (F) Hill plot of the binding data. (G) Enhancement in fluorescence intensity (ΔF) due to ANS bound to Rec1-resilin. (H) Effect of type and level of crowder on the Stern-Volmer quenching constants, KSV. a.u., arbitrary units.