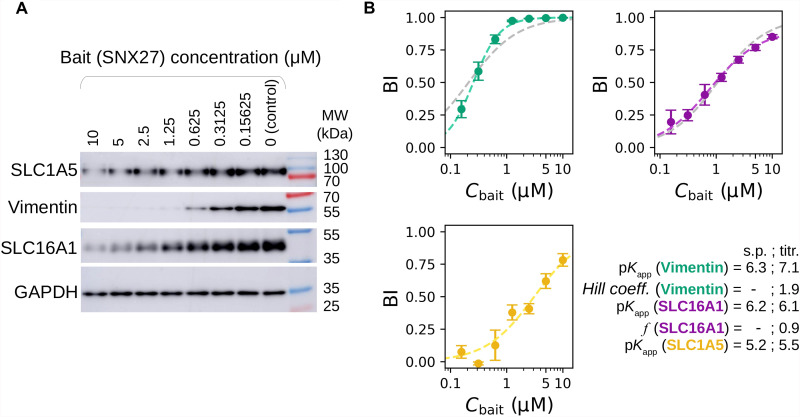
Fig. 5. Exploring binding mechanisms of SNX27 interactions with nHU.
(A) Results of nHU-WB titration experiments performed with full-length SNX27 bait. (B) Endogenous full-length SLC1A5 (yellow), SLC16A1 (purple), and Vimentin (green) prey depletions were quantified using specific antibodies. BI values of all prey were first fitted with a hyperbolic binding equation. In the case of SLC1A5, good fit was achieved with a simple hyperbolic binding equation (yellow dashed line). In the case of SLC16A1, an imperfect fit was achieved with a simple hyperbolic binding equation (gray dashed line), and more accurate fit was found when 10% inactive fraction was assumed (purple dashed line, f = 0.9, see Materials and Methods for further details). In the case of Vimentin, a low-quality fit was achieved with a simple hyperbolic binding equation (gray dashed line), and near-perfect fit was obtained using the Hill equation (green dashed line). Equilibrium affinities of single-point measurements (s.p.) and parameters determined from the titration experiments (titr.) are indicated in the bottom-right corner. BI values were determined on the basis of three replicates. See fig. S6 and table S1 for additional data.