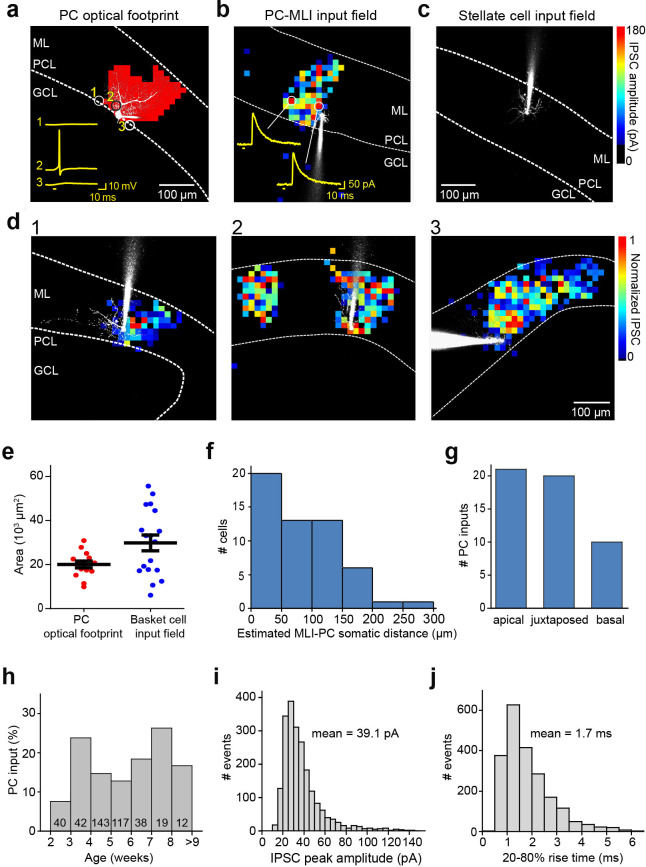
Figure 3. Spatial organization of PC-to-MLI circuit.
(a) Scanning a brief laser light spot (405 nm, 3 µW, 4 ms duration) across a cerebellar slice revealed locations (red pixels) where photostimulation evoked action potentials in a ChR2-expressing, dye-filled PC (white). Numbered traces (yellow) in the inset show membrane potential changes produced when laser spot was located at the indicated pixels. Bar below traces indicates time of light flashes. Action potentials were evoked when the light spot was located over the cell body and entire dendritic region of the PC. ML, molecular layer; PCL, Purkinje cell layer; GCL, granule cell layer. (b) Map of inhibitory input from a presynaptic PC to a postsynaptic PC-MLI was created by correlating light spot location with amplitude of inhibitory postsynaptic currents (IPSCs) evoked by focal photostimulation of PCs. IPSC amplitudes are encoded in the pseudocolor scale shown at right. Traces show IPSCs evoked at the indicated locations and bars indicate time of light flashes. (c) No inhibitory input from PCs was detected in stellate cells. (d) Varied spatial organization of PC inputs to PC-MLIs. Maps illustrate IPSC amplitudes (pseudocolor scale at right) evoked in three different dye-filled PC-MLIs (white) shown in panels 1–3. (e) Comparison of input field area of PC-MLIs (blue, n=18) with PC optical footprints (red, n=14). (f) Distance between postsynaptic PC-MLIs and presynaptic PCs. PC soma location was estimated from the center of the input field, projected down to the PCL. (g) Orientation of postsynaptic PC-MLIs, relative to presynaptic PCs. (h) Connectivity between PCs and PC-MLIs measured at different ages. Numbers inside bars indicate sample sizes. (i) Distribution of amplitudes of IPSCs evoked by focal photostimulation of PCs. Holding potential was –40 mV. (j) Distribution of rise times of light-evoked IPSCs. MLI, molecular layer interneuron; PC, Purkinje cell.