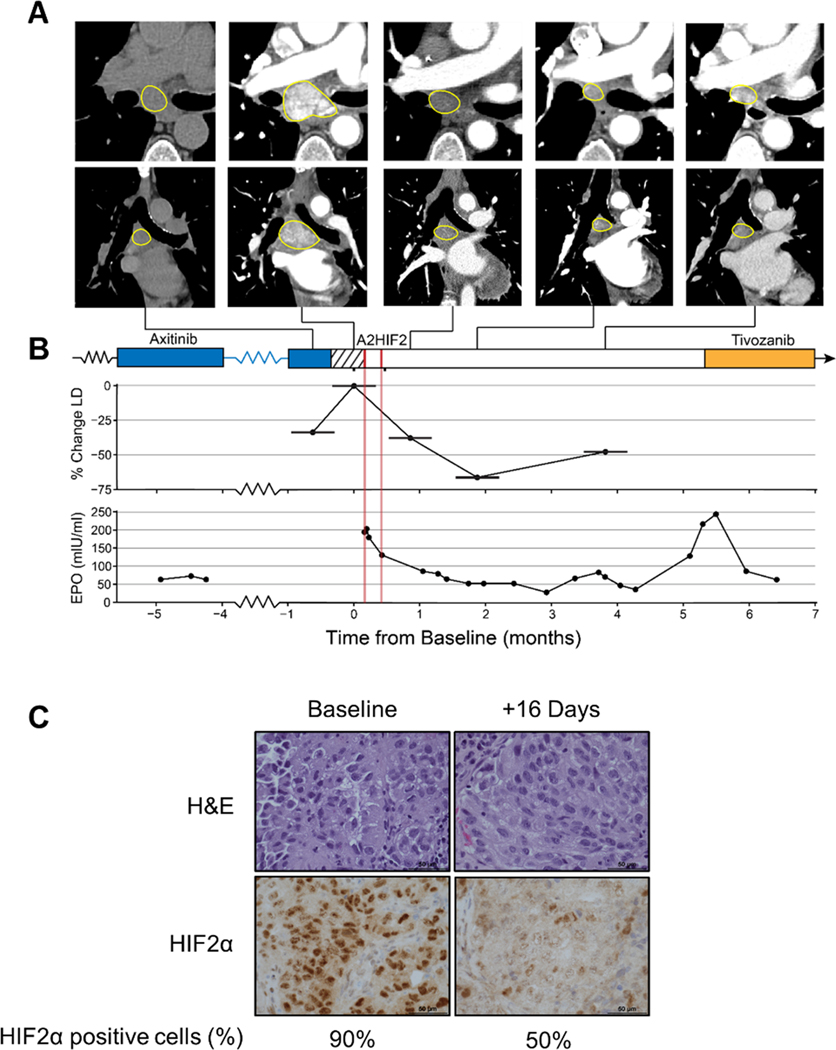
Fig. 6. HIF2α downregulation, Epo suppression and tumor growth inhibition by siHIF2 in clinical trial participant (106–00C).
A. Axial (top) and coronal (bottom) CT images of subcarinal lymph node target lesion showing rapid progression during 2-week washout period and deep prolonged response after two doses of A2HIF2. While the first CT scan was performed without iodinated contrast, marked decrease in lymph node enhancement between baseline (second) CT and subsequent CTs illustrates profound antiangiogenic effect. B. Treatment timeline, tumor change (LD, longest diameter) and erythropoietin (EPO) levels prior to and following A2HIF2 administration (red lines). C. H&E and HIF2α immunohistochemistry of lymph node biopsy at baseline and 16 days after treatment onset with percent of tumor cells positively staining for HIF2α.