Figure 7. CWI1-2 exhibits potent anti-leukemia efficacy in vitro and in vivo.
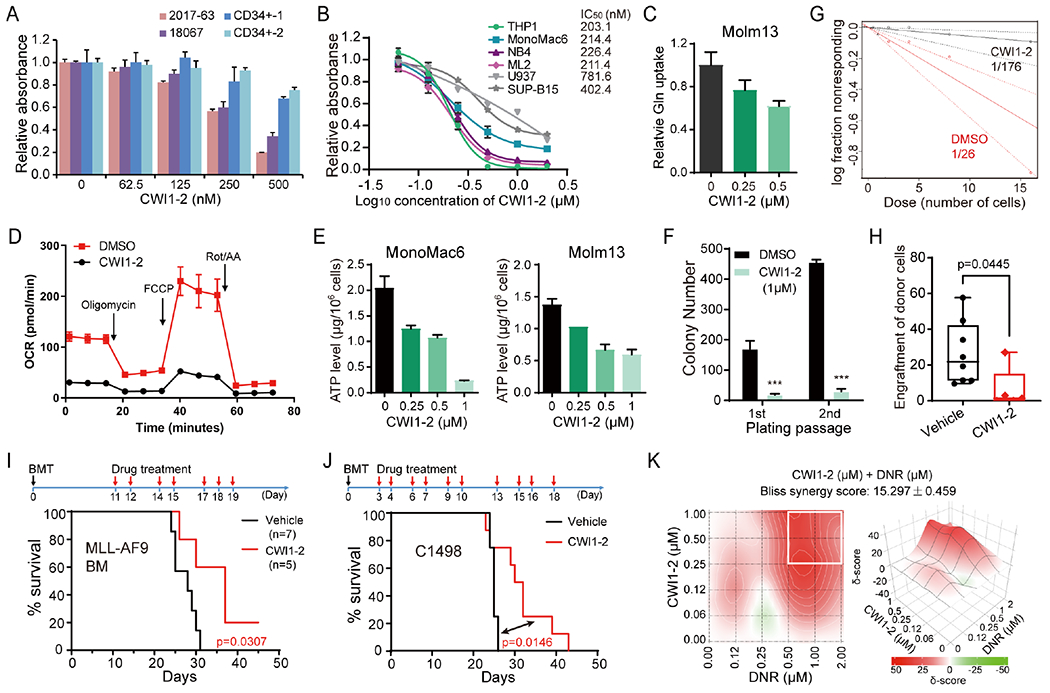
(A) MTT assays of patient-derived AML cells or CD34+ CB cells from healthy controls in the presence of CWI1-2. (B) MTT assays and the IC50 values of CWI1-2 in leukemia cell lines after 48 hours of treatment. (C-E) AML cell lines were treated with indicated concentration of CWI1-2 for 24 hours and subjected to the examination of glutamine uptake (C), OCR (D), and ATP levels (E). (F) Effect of CWI1-2 on the colony-forming/replating capacity of MA9-immortalized mouse HSPCs. (G) The in vitro LDA using MA9-immortalized mouse HSPCs. (H) Engraftment of donor cells in the PB of recipient mice after transplanting with BM cells from MA9-induced leukemic mice and subsequent i.v. injection of CWI1-2 or vehicle control. (I) Kaplan-Meier survival curves of recipient mice in (H). (J) Kaplan-Meier survival curves of mice transplanted with C1498 cells and treated with CWI1-2 or vehicle control. Days of BMT and drug treatment were shown in the upper panels in (I) and (J), with black arrows indicating BMT while red arrows indicating drug treatment. (K) Synergistic effect of CWI1-2 with DNR on inhibition of the survival/growth of C1498 cells as determined by the Bliss independent model. Drug combinations with the strongest synergistic effects are outlined with white squares. δ-score represents the percentage of response beyond expectation due to drug interactions. Data are represented as mean ± SD. ***p < 0.001; t test. See also Figure S7.
