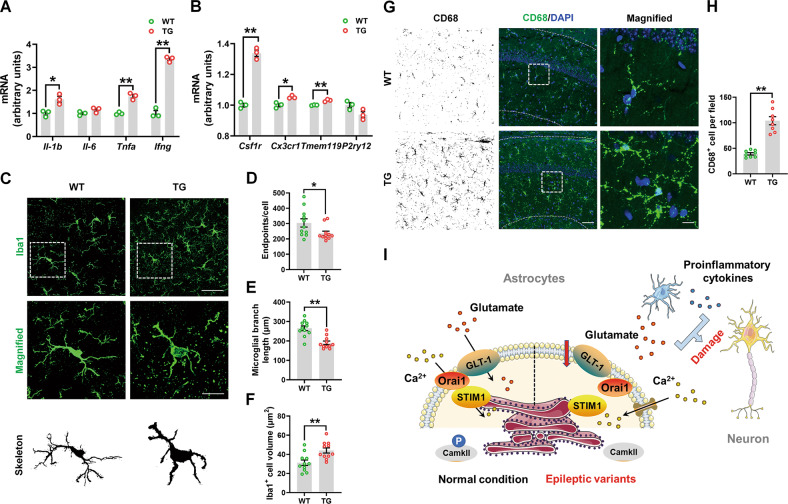
Fig. 8. G82R/L85P variant knock-in mice had neuroinflammation.
A, B The mRNA expression levels of Il-1b, Il-6, Tnfa, Ifng, Csf1r, Cx3cr1, Tmem119, and P2ry12 were determined by qRT-PCR. C Immunofluorescence staining of Iba1-positive cells in the hippocampus of WT and TG mice. Scale bar, 20 μm. Magnified Iba1-positive cells are shown in the middle column of panel (C), and their skeletal images are shown in the bottom column of panel (C). Scale bar, 5 μm. D–F Quantification of endpoints per cell, branch length, and the volume of Iba1-positive cells in panel (C). n = 11 per group. G Immunofluorescence staining of CD68-positive cells in the hippocampus of WT and TG mice. Scale bar, 50 μm. Magnified CD68-positive cells are shown in the right column of panel (G). Scale bar, 10 μm. H Quantification of the numbers of CD68-positive cells in panel (G). n = 8 per group. I Schematic model of the study. In the normal condition, GLT-1 is responsible for glutamate uptake and interacts with STIM1/Orai1 to maintain the Ca2+ refilling and phosphorylation of CaMKII in the ER. However, epileptic variants (G82R, L85P, and P289R) decrease the expression and function of GLT-1 and inhibit STIM1/Orai1-mediated SOCE together with the phosphorylation of CaMKII. Glutamate excitotoxicity and microglia-released proinflammatory cytokines damage the hippocampal neurons and induce epilepsy. Results are expressed as mean ± SD. **P < 0.01, **P < 0.05 vs. WT. Statistical significance was determined using Student’s t test.