Table 8.
One-pot synthesis of tetrahydrobenzo[b]pyran with aldehyde, malononitrile and dimedone catalyzed by HMS/Pr-PTSC-Cua.
| Entry | Product | Time (min) | Yield (%)b | TOF (h-1) | M.p (°C) | Ref |
|---|---|---|---|---|---|---|
| 1 |
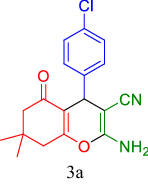
|
30 | 97 | 7698 | 209–211 | 25 |
| 2 |
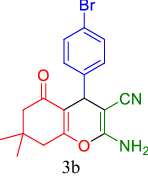
|
35 | 96 | 6568 | 198–200 | 42 |
| 3 |
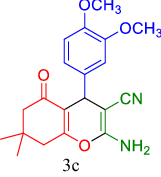
|
70 | 81 | 2747 | 176–178 | 43 |
| 4 |
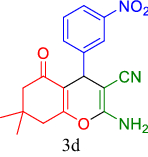
|
40 | 93 | 5508 | 210–212 | 44 |
| 5 |
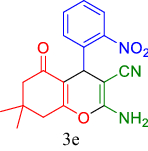
|
30 | 96 | 7619 | 228–231 | 19 |
| 6 |

|
80 | 80 | 2386 | 194–196 | 45 |
| 7 |
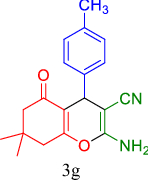
|
65 | 83 | 3049 | 210–212 | 45 |
| 8 |
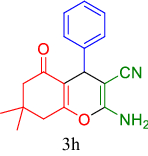
|
40 | 95 | 5626 | 226–230 | 46 |
| 9 |
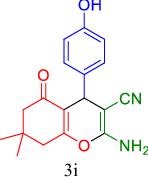
|
50 | 88 | 4207 | 205–207 | 47 |
| 10 |
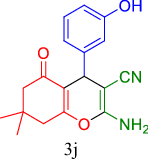
|
60 | 85 | 3373 | 230–234 | 43 |
| 11c |
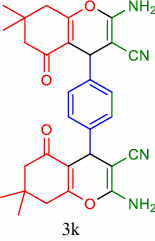
|
15 | 99 | 15,714 | 256–260 | 48 |
aReaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), dimedone (1 mmol), HMS/Pr-PTSC-Cu (0.004 g) in EtOH at room temperature.
bPure yield.
cReaction conditions: aldehyde (1 mmol), malononitrile (2 mmol), dimedone (2 mmol), HMS/Pr-PTSC-Cu (0.008 g) in EtOH at room temperature.
