Abstract
Abstract
Background
Altered expression of the complement component C4A gene is a known risk factor for schizophrenia. Further, predicted brain C4A expression has also been associated with memory function highlighting that altered C4A expression in the brain may be relevant for cognitive and behavioral traits.
Methods
We obtained genetic information and performance measures on seven cognitive tasks for up to 329 773 individuals from the UK Biobank, as well as brain imaging data for a subset of 33 003 participants. Direct genotypes for variants (n = 3213) within the major histocompatibility complex region were used to impute C4 structural variation, from which predicted expression of the C4A and C4B genes in human brain tissue were predicted. We investigated if predicted brain C4A or C4B expression were associated with cognitive performance and brain imaging measures using linear regression analyses.
Results
We identified significant negative associations between predicted C4A expression and performance on select cognitive tests, and significant associations with MRI-based cortical thickness and surface area in select regions. Finally, we observed significant inconsistent partial mediation of the effects of predicted C4A expression on cognitive performance, by specific brain structure measures.
Conclusions
These results demonstrate that the C4 risk locus is associated with the central endophenotypes of cognitive performance and brain morphology, even when considered independently of other genetic risk factors and in individuals without mental or neurological disorders.
Key words: Cognition, immune system, major histocompatibility complex, mental health, psychiatric disorder, schizophrenia
Introduction
The major histocompatibility complex (MHC) is located on chromosome 6 and is implicated in a number of autoimmune diseases (Howson, Walker, Clayton, & Todd, 2009; Kamitaki et al., 2020; Raychaudhuri et al., 2012). In addition, genetic variants within this region are consistently associated with risk of schizophrenia (International Schizophrenia Consortium et al., 2009; Pardiñas et al., 2018; Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium, 2011; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014; Shi et al., 2009; Stefansson et al., 2009). These associations corroborate serological studies which identified altered levels of inflammatory markers in schizophrenia patients, including complement proteins (Hakobyan, Boyajyan, & Sim, 2005; Laskaris et al., 2019; Maes et al., 1997; Mayilyan, Arnold, Presanis, Soghoyan, & Sim, 2006; Mayilyan, Dodds, Boyajyan, Soghoyan, & Sim, 2008a; Mayilyan, Weinberger, & Sim, 2008b). These findings suggest the involvement of an immune component in psychiatric disorders such as schizophrenia.
In order to better understand the mechanisms underlying the MHC genetic association with schizophrenia, a fine-mapping molecular investigation of the region was conducted and identified that variants within the complement component 4 (C4) gene locus are responsible for at least part of the association signal (Sekar et al., 2016). The C4 protein is one of a number of proteins that make up the complement system (Charles, Janeway, Travers, Walport, & Shlomchik, 2001), part of the innate immune system. Complement components were initially shown to modulate neurogenesis in murine primary cortical cell cultures (van Beek et al., 2001). Further investigation of the role of complement components in the central nervous system of genetically modified mice identified its major role in modulating synaptic plasticity (Hong et al., 2016; Stephan, Barres, & Stevens, 2012; Stokowska et al., 2017; Vasek et al., 2016). More recently, complement components were implicated in neuronal migration (Gorelik et al., 2017) and apoptosis (Niculescu et al., 2004) in the central nervous system. Additional evidence for the activity of the complement system in the brain, and its involvement in the pathogenesis of schizophrenia is summarized in recent reviews (Druart & Le Magueresse, 2019; Nimgaonkar, Prasad, Chowdari, Severance, & Yolken, 2017; Tenner, Stevens, & Woodruff, 2018; Woo, Pouget, Zai, & Kennedy, 2019).
The C4 gene is present as one of two isotypes (C4A and C4B) and the structural variation between these isotypes, as well as their copy number, was shown to significantly alter the expression level of C4 in post-mortem brain tissue (Sekar et al., 2016). A model of this relationship can be used to predict C4A gene expression in the brain based on an individual's genotype. Using this procedure, predicted C4A gene expression was associated with risk of schizophrenia in an independent sample (Sekar et al., 2016). Finally, C4 proteins localized to the synapses in post-mortem human brains, and C4 was also demonstrated to modulate synaptic pruning in mice (Sekar et al., 2016), and human-derived neural cultures (Sellgren et al., 2017, 2019).
Independent of these findings, variants within the MHC region were also associated with cognitive performance (Athanasiu et al., 2017; Donohoe et al., 2013; Zhang, Lv, Fan, Tang, & Yi, 2017) and brain structure (Walters et al., 2013) in patients with schizophrenia. Based on these studies, Donohoe et al. (2018) showed that increased predicted C4A expression was associated with poorer performance in memory recall measures in a cohort of psychosis patients and healthy controls, as well as in patients only. The direction of effect in control participants was similar to that observed in patients, however, the effect size was smaller and non-significant. In addition, they demonstrated that higher predicted C4A expression was associated with lower cortical activity in the middle temporal cortex during visual processing in healthy participants (Donohoe et al., 2018). In support of these findings, complement-dependent synapse elimination was recently identified as a mechanism for memory loss (Wang et al., 2020). These results highlight that C4A expression in the brain may be associated with cognitive and behavioral traits not only in patients with psychiatric disorders but also in healthy individuals.
Based on this, our primary aim was to investigate if predicted brain C4A expression is associated with cognitive performance in a large adult population-based sample (UK Biobank), without mental or neurological disorders. We hypothesized that higher predicted C4A expression would be associated with lower cognitive performance, however, we did not start with any a priori assumptions regarding the specific cognitive tasks investigated. Our secondary aims were to investigate if predicted brain C4A expression is associated with differences in brain structure and if observed effects on cognitive performance may be mediated by C4A-associated differences in brain structure.
Methods
The UK Biobank cohort
The UK Biobank cohort and available data are described elsewhere (Bycroft et al., 2018). Briefly, the UK Biobank project is a prospective cohort study with genetic and phenotypic data collected on approximately 500 000 individuals from across the UK. Multimodal imaging assessments are underway, with magnetic resonance imaging (MRI) of the brain currently available for a subset of individuals (Miller et al., 2016). All data used in this study were obtained from the UK Biobank (http://www.ukbiobank.ac.uk) through application 27412.
We limited the cohort to 409 629 Caucasian individuals (Datafield-22006). This subset is defined as those individuals who self-identified as ‘White British’ and that had similar genetic ancestry based on a principal component analysis (online Supplementary Fig. S1). Individuals with a diagnosed mental or neurological disorder were excluded (Datafields-41202,41204; F/G codes). One from each pair of individuals with a kinship coefficient above 0.053 was also removed prior to analyses (Datafield-2201122012).
The final cohort sample size, after exclusions, with available genetic data was 329 773 (median age 59, range: 40–74). The sample included 152 966 men (median age 59, range: 40–74) and 176 807 women (median age 58, range: 40–71).
All participants provided informed consent prior to enrolment. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Genotyping and quality control
Genotyping of the UK Biobank cohort was performed on two similar arrays. Approximately 50 000 samples were genotyped on the UK BiLEVE array and the remaining 450 000 samples were genotyped on the UK Biobank Axiom array. Further details regarding genotyping and quality control procedures for the UK Biobank are well documented (Bycroft et al., 2018).
Imputation of C4 structural variation and genetically predicted C4a expression
Direct genotypes for variants (n = 3213) within the MHC region were used to impute C4 structural variation. This analysis was performed using the 222 haplotype-integrated variant and C4 reference panel (Sekar et al., 2016). The distribution of C4 structural variants was similar to previously described (online Supplementary Table S1) (Sekar et al., 2016; Kamitaki et al., 2020). The imputed C4 structural alleles were then used to determine C4 isotype (C4A, C4B, C4L, and C4S) copy numbers. Here C4A and C4B refer to the two isotypes of the C4 gene, while C4L and C4S refer to ‘long’ and ‘short’ forms of the gene due to the presence or absence of a human endogenous retroviral (HERV) insertion, respectively. We calculated values for the predicted expression of the C4A gene in human brain tissue, based on the previously identified relationship between C4 isotype copy number and C4A gene expression (Sekar et al., 2016). The predicted C4A expression values ranged between 0 and 2.35 (mean = 1.08, standard deviation = 0.36) (online Supplementary Fig. S2). A summary of this methodology is presented in Fig. 1. Predicted C4B expression values were calculated following a similar approach. Predicted C4A and C4B expression values were used for association with cognitive tasks and brain imaging measures since these variables allow for use of standard linear regression analyses instead of ordinal regression using structural variants.
Fig. 1.
A schematic diagram of the methodology used to obtain predicted expression values for the C4A gene within brain tissue, as described by Sekar et al. (2016). First, (a) individual genotypes are determined and SNP haplotypes are then inferred from this data. (b) The SNP haplotypes can be grouped into haplogroups and each haplogroup corresponds to a specific C4 locus structure. Four of these structures are common (represented here) and 11 are less common (<10% frequency combined). HERV, human endogenous retroviral insertion. (c) Structures with higher copy numbers of C4A and C4L (both C4AL and C4BL) isotypes show higher C4A expression in brain tissue. (d) C4A gene expression can be predicted based on the data outlined in panels A–C. AL, AS, BL, and BS refer to the copy number of each of these isotypes in the C4 locus structure. Structures containing the AS combination are omitted from panels A to C since they are rare, with a frequency of approximately 1% (online Supplementary Table S1) (Sekar et al., 2016). This figure is a schematic and was not generated from actual genotype, expression or other data.
Cognitive tasks
We obtained performance measures on seven cognitive tasks from the UK Biobank, and processed them as previously described (Kendall et al., 2017, 2019). Briefly, measures for analysis included the Pairs Matching task (episodic memory, Datafield-399, outcome: total number of errors), the Reaction Time task (simple processing speed, Datafield-20023, outcome: mean reaction time to correct responses), the Fluid Intelligence test (reasoning and problem solving, Datafield-20016, total number of correct answers), the Digit Span task (numeric working memory, Datafield-4282, outcome: maximum number of digits remembered), the Symbol Digit Substitution task (complex processing speed, Datafield-20195, outcome: number of correct substitutions), and the Trail Making A and B tasks (visual attention, Datafields-20156,20157, outcome: time taken to complete these tests). All data were recoded so that higher scores indicate better performance. The number of participants that completed each of these performance measures, with available predicted C4A and C4B expression values and brain imaging data, is provided in Table 1.
Table 1.
Numbers of participants that completed each of the seven cognitive tasks, with available predicted C4A and C4B expression values and brain imaging data
| Cognitive task | With predicted C4A and C4B expression (n) | With C4A and C4B expression Values and Brain Imaging Data (n) |
|---|---|---|
| Pairs matching | 329 465 | 21 989 |
| Reaction time | 327 815 | 22 064 |
| Fluid Intelligence | 106 633 | 7484 |
| Digit span | 34 171 | 2195 |
| Symbol Digit Substitution | 81 444 | 11 696 |
| Trail Making A | 71 933 | 10 427 |
| Trail Making B | 71 931 | 10 427 |
Image acquisition and processing
Imaging assessments were conducted at three centers, using the same hardware, software and protocols. A detailed description of the processes for data acquisition, processing and quality control is available (Alfaro-Almagro et al., 2018). The data release from UK Biobank used in this study included 33 003 participants. C4A and C4B expression values were predicted for 27 087 of these participants.
We processed T1-weighted MRI scans from all individuals using the standardized recon-all pipeline of FreeSurfer (Fischl et al., 2002; Fischl, 2012). Furthermore, for each scanner site, we regressed age and sex from the Euler number of both left and right hemispheres and individuals whose Euler numbers were less than 3 standard deviations below the residualized Euler numbers were excluded as outliers (n = 618) (Kaufmann et al., 2019). Analyzed brain imaging measures included surface area and mean thickness of 34 cortical regions, total cortical surface area, and mean cortical thickness, the volumes of seven sub-cortical regions, and total intracranial volume (ICV). The total surface area, thickness or volume of each region was calculated by summing the right and left hemispheres.
Statistical analyses
To determine the relationship between cognitive performance and predicted C4A and C4B expression, we performed linear regression analyses with each cognitive task as the outcome variable, predicted C4A or C4B expression as the predictor variable and common covariates, which included age, age-squared, sex, genotyping batch, the first 10 genetic principal components and educational attainment. A summary of the effects of these covariates on C4A and C4B expression is provided in online Supplementary Table S2. Age-squared was included since this allows the model to accommodate a non-linear relationship between age and the outcome variable if one exists. Educational attainment was determined by the highest qualification obtained by each individual at the time of assessment (Datafield-6138). No significant associations were identified between predicted C4B expression and cognitive tasks (online Supplementary Table S3), and therefore predicted C4B expression was not tested for associations with brain imaging measures.
To investigate the relationship between brain imaging measures and predicted C4A expression values, brain imaging measures were first normalized in R 3.5.0 by an inverse normal transformation of the residual of linear regression on the phenotype correcting for covariates, as previously described (Sønderby et al., 2018). This transformation results in normally distributed covariate-corrected values that were used for downstream analysis. Covariates included the common covariates mentioned above as well as Euler number (Rosen et al., 2018). Regional measures of surface area and mean thickness were corrected for total cortical surface area and total mean cortical thickness, respectively. Subcortical volumes were corrected for ICV.
To determine the association between of predicted C4A expression and brain structure, we performed linear regression analyses with the covariate-corrected brain imaging measure as the outcome and predicted C4A expression as the predictor variable in the model.
Finally, to determine if the effects of predicted C4A expression on cognitive tasks were mediated by brain imaging measures, additional linear regression analyses were performed with each cognitive task as the outcome variable, predicted C4A expression, a regional non-covariate-corrected brain imaging measure and covariates. Covariates included the common covariates, Euler number (Rosen et al., 2018), and educational attainment. Regional measures were corrected for using global measures as described above. Mediation analysis was then performed using the R package mediation v4.4.6, using the bootstrapping method and 5000 simulations per test (Writing Committee for the ENIGMA-CNV Working Group et al., 2019). All significant results are also shown in the context of a mediation model (Fig. 2). A previous study investigating the effects of brain imaging measures on cognitive performance in the UK Biobank has shown significant positive correlations between all of the brain imaging measures included in this study and increased cognitive performance (Cox, Ritchie, Fawns-Ritchie, Tucker-Drob, & Deary, 2019). Those results correspond to path b in the mediation analyses performed in this study (Fig. 2).
Fig. 2.
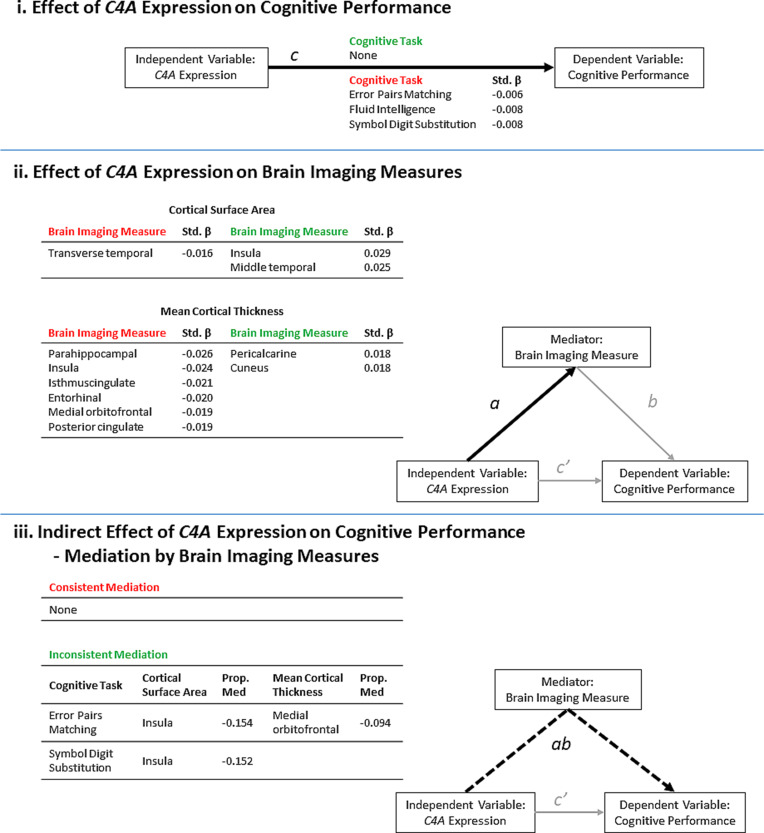
A summary of the results from the significant (FDR <0.05) linear regression models of predicted C4A expression values on cognitive performance and brain imaging measures. The results are presented in the context of a mediation model. (i) Higher predicted C4A expression was significantly associated with the results from three cognitive tasks. Path c = Cognitive task ~ C4A expression (ii) Predicted C4A expression was significantly associated with some measures of cortical surface area and cortical thickness. Path a = Brain imaging measure ~ C4A expression. (iii) A summary of the brain imaging measures identified to significantly mediate the effect of predicted C4A expression on cognitive performance. Path ab = Cognitive task ~ C4A expression mediated by brain imaging measures. The proportion of the total effect (Panel i, Path c) mediated by changes in the corresponding brain imaging measure is shown (Prop. Med = ab/c). Negative proportion values indicate inconsistent mediation. Inconsistent mediation occurs when the direction of effect of the direct effect (c’) and the indirect effect (ab) is in the opposite direction. The standardized β (Std. β) is shown to indicate the size and direction of effect of higher C4A expression on each outcome measure. The green and red headers indicate an increase or decrease in each outcome measure, respectively.
Since sex-specific C4A risk effects were recently identified (Kamitaki et al., 2020), additional analysis was performed as above with the inclusion of an interaction term between C4A expression and sex (online Supplementary Table S4). The number of male and female participants that completed each of the performance measures, with available predicted C4A and C4B expression values and brain imaging data, is provided in online Supplementary Table S5.
The distributions of residuals from all models were examined and determined to be normal indicating that linearity assumptions were not violated. Effect sizes reported are the standardized estimates of beta (β) from the linear regressions. The partial correlation coefficient (r) was computed from the t-statistics for the main cognitive and brain structure analyses (online Supplementary Tables S6–S9). The distribution of values for significantly associated cognitive performance tests and brain imaging measures were plotted against ‘binned’ predictions of C4A expression levels (online Supplementary Figs S3–S5) and analysis of variance tests and post-hoc Tukey tests were used to determine the differences between these ‘bins’ (online Supplementary Tables S10–S12). Empirical p values were converted to False Discovery Rate (FDR) q-values using the R package qvalue v2.14.1. FDR was computed independently for the analyses of cognitive tests (n = 7), brain morphology (n = 79) and mediation (n = 33). Results were considered significant if FDR <0.05. Plots were generated using R library ggplot2 v2.2.1 (Wickham, 2009, p. 2) and the R package ggseg v1.5.1.
Results
Effect of C4a expression on cognitive performance
Predicted C4A expression was significantly (FDR < 0.05) associated with three of the seven cognitive tests (Fig. 2i, Table 2, online Supplementary Table S6). Specifically, higher predicted C4A expression was associated with reduced cognitive performance in the pairs matching (Std. β = −0.006, t-value = −3.28, FDR = 0.009), fluid intelligence (Std. β = −0.008, t-value = −2.86, FDR = 0.032), and symbol digit substitution (Std. β = −0.008, t-value = −2.75, FDR = 0.043) cognitive tasks. Analysis of the association between predicted C4A expression and cognitive performance measures indicates a linear relationship, not a distinct range of expression above or below which the observed changes occur (online Supplementary Table S10). No significant C4A–sex interactions were identified for any of the cognitive tests (online Supplementary Table S4).
Table 2.
A summary of the results from the significant linear regression models of predicted C4A expression values on cognitive performance
| Phenotype and Covariates | R | Std. β | Std. Error | Uncorrected p value | FDR |
|---|---|---|---|---|---|
| Pairs matching | |||||
| C4A expression | −0.006 | −0.006 | 0.003 | 1.046 × 10−3 | 9.212 × 10−3 |
| Age | −0.128 | −0.135 | 0.675 | <1 × 10−300 | <1 × 10−300 |
| Sex (Male) | 0.016 | 0.016 | 0.002 | 4.204 × 10−20 | 5.028 × 10−19 |
| Fluid Intelligence | |||||
| C4A expression | −0.009 | −0.008 | 0.016 | 4.174 × 10−3 | 0.032 |
| Age | −0.011 | −0.011 | 0.001 | 2.415 × 10−4 | 2.319 × 10−3 |
| Sex (Male) | 0.066 | 0.059 | 0.012 | 1.209 × 10−101 | 2.838 × 10−100 |
| Symbol Digit Substitution | |||||
| C4A expression | −0.010 | −0.008 | 0.040 | 5.930 × 10−3 | 0.043 |
| Age | −0.451 | −0.455 | 0.002 | <1 × 10−300 | <1 × 10−300 |
| Sex (Male) | −0.007 | −0.006 | 0.029 | 5.477 × 10−2 | 0.248 |
All models also included age squared, educational attainment, genotyping batch, and the first 10 genetic principal components as covariates (data not shown). r = Partial correlation coefficient. Std. β = Standardized β. Std. Error = Standard Error.
Effect of C4a expression on brain imaging measures
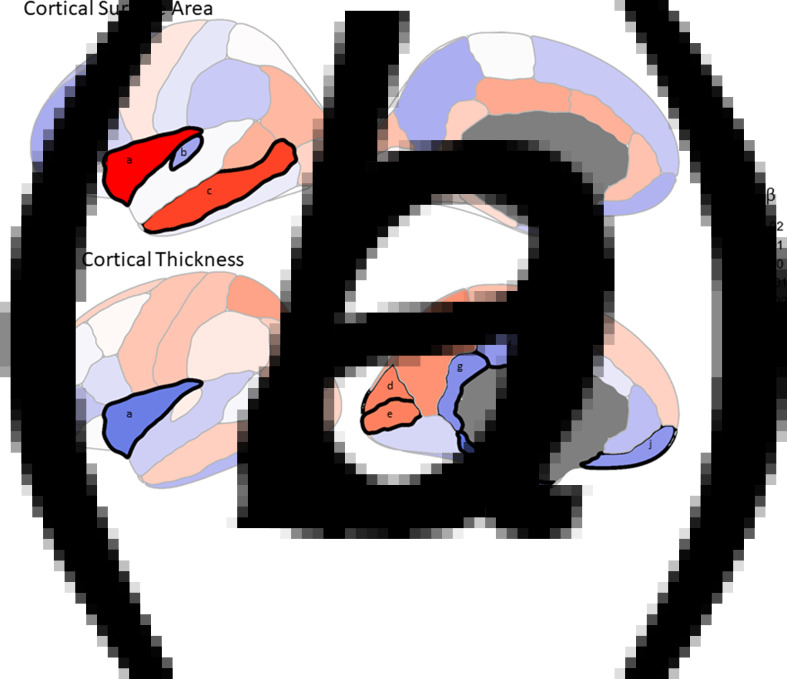
Predicted C4A expression was significantly (FDR < 0.05) associated with three cortical surface area measures (Fig. 3a, online Supplementary Table S7). Specifically, higher C4A expression was associated with reduced surface area for the transverse temporal measure (Std. β = −0.016, t-value = −2.68, FDR = 0.045), and increased surface area of the insula (Std. β = 0.029, t-value = 4.70, FDR = 1.735 × 10−4), and middle temporal (Std. β = 0.025, t-value = 4.15, FDR = 7.458 × 10−4) measures, respectively (Fig. 2ii).
Fig. 3.
The effect of C4A expression on regional measures of (a) cortical surface area and (b) mean cortical thickness. The colors correspond to the standardized β (Std. β) coefficient for each brain region from the linear regressions. Black demarcations around a brain region indicate that it passes the multiple comparisons–corrected significance threshold of FDR <0.05. a, Insula. b, Transverse temporal. c, Middle temporal. d, Cuneus. e, Pericalcarine. f, Posterior cingulate. g, Isthmuscingulate. h, Parahippocampal. i, Entorhinal. j, Medial orbitofrontal.
When considering mean cortical thickness, predicted C4A expression was significantly associated with eight measures, the majority (6 of 8) of which were negatively associated with C4A expression (Fig. 3b, online Supplementary Table S8). Specifically, the parahippocampal (Std. β = −0.026, t-value = −4.22, FDR = 7.458 × 10−4), insula (Std. β = −0.024, t-value = −3.96, FDR = 1.277 × 10−3), isthmuscingulate (Std. β = −0.021, t-value = −3.38, FDR = 9.865 × 10−3), entorhinal (Std. β = −0.020, t-value = −3.22, FDR = 0.014), medial orbitofrontal (Std. β = −0.019, t-value = −3.14, FDR = 0.016) and posterior cingulate (Std. β = −0.019, t-value = −3.08, FDR = 0.017) measures (Fig. 2ii).
No significant associations were identified between predicted C4A expression and subcortical volumes. In addition, no other regional brain measures, or global measures including total cortical surface area, total mean cortical thickness and ICV, were significantly associated with predicted C4A expression (online Supplementary Tables S7–S9). As with cognitive performance, further analysis of the association between predicted C4A expression and regional brain imaging measures indicates that this relationship is linear and that there is not a distinct range of expression above or below which the observed changes occur (online Supplementary Tables S11–S12 and online Supplementary Figs S3–S5). Hemisphere-specific results are provided in the supplement (online Supplementary Tables S13–S15). A summary of the effects of predicted C4A expression on brain imaging measures, and how these results are incorporated into the mediation analyses are shown in Fig. 2ii.
No significant C4A–sex interactions were identified for any brain imaging measures (online Supplementary Tables S16–S18).
Indirect effect of C4a expression on cognitive performance – mediation by brain imaging measures
Mediation analyses highlighted that increases in insula surface area and medial orbitofrontal thickness are linked to significant (FDR < 0.05) inconsistent mediation of the effect of higher predicted C4A expression on two measures of cognitive performance (Fig. 2iii), i.e. the changes in brain imaging measures partially suppress the negative effects of higher C4A expression on cognitive performance. None of the included brain imaging measures was identified as significant mediators of the effect of predicted C4A expression on fluid intelligence scores (online Supplementary Table S19).
Discussion
Here we identified novel significant associations between predicted brain C4A expression and cognitive performance in a large adult volunteer sample of individuals without mental or neurological disorders. Additionally, we showed that predicted C4A expression was significantly associated with regional cortical thickness and surface area. Further analysis of these associations revealed that their relationships are linear, and that there is no distinct threshold value for predicted C4A expression, highlighting that multiple factors likely influence cognition and brain morphology in these individuals within the normal range. Finally, we identified significant inconsistent partial mediation of the effects of C4A expression on cognitive performance, by specific brain imaging measures. This indicates that the differences observed in brain morphology may help to protect against C4A-associated cognitive deficits. In addition, our observations of lower cognitive performance and differences in brain imaging measures are highly unlikely to be secondary to any mental or neurological disorders or the treatment thereof since we excluded individuals with diagnosed mental or neurological disorders, and the remaining individuals within the UK Biobank tend to be healthier than the general population (Fry et al., 2017).
The main finding of this study is the negative association between predicted C4A expression in the brain and episodic memory (Pairs Matching task), reasoning and problem solving (Fluid Intelligence test) and complex processing speed (Symbol Digit Substitution task). Our regression modelling shows that the effects of predicted C4A expression, in some instances, are comparable in size to known modifiers of cognitive performance, such as with age for fluid intelligence and with sex for symbol digit substitution (Table 2). As expected, when comparing these effect sizes to those of rare copy number variants (CNVs) with known cognitive effects, a study on the same UK Biobank participants showed that most such CNVs had a greater effect on cognitive performance than that observed for predicted C4A expression in this study (Kendall et al., 2019). These results are in line with previous findings, that higher predicted C4A expression is associated with poorer performance in memory recall measures in psychosis patients (Donohoe et al., 2018) and that the complement system modulates memory loss (Wang et al., 2020), and further demonstrate that these effects are present within unaffected individuals. Predicted C4B expression was not associated with cognitive performance, the effect of the C4 locus was limited to C4A as suggested by previous findings (Donohoe et al., 2018; Sekar et al., 2016). Moreover, we did not identify any strong correlation between schizophrenia polygenic risk score and predicted C4A expression (data not shown), implying that predicted C4A expression is not a proxy for schizophrenia polygenic risk in the UK Biobank sample analyzed.
Cognitive impairments reliably distinguish between schizophrenia patients and healthy controls, with large effect sizes in meta-analyses (Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009). Moreover, similar observations, with smaller effects, for measures of processing speed, attention and memory have also been identified when comparing first-degree relatives of schizophrenia patients to healthy controls (Hou et al., 2016). At a molecular level, shared common variants contributing to both schizophrenia risk and cognitive performance have also been identified (Smeland et al., 2019). These studies highlight cognitive impairment as a core heritable feature of schizophrenia (Barch & Ceaser, 2012; Bora, Yücel, & Pantelis, 2010), which may manifest in both affected patients and healthy individuals with some genetic burden for the disorder. Cognitive deficits have been associated with poorer functional outcomes regardless of age, sex or chronicity of the disorder (Fett et al., 2011). This lead to the suggestion that common mechanisms might modulate individual differences within these cognitive domains, e.g. related to the structure, function and/or connectivity of prefrontal, parietal, cingulate and insula brain regions (Barch & Ceaser, 2012). Our brain imaging results highlight that C4A expression may potentially act as one of the causative factors in such mechanisms.
We identified significant associations between predicted C4A expression and cortical surface area and/or mean cortical thickness within temporal, cingulate and insula cortex, amongst others (Figs 1ii and 2). In line with previous observations of structural brain abnormalities in patients with schizophrenia (Cobia, Csernansky, & Wang, 2011; van Haren et al., 2011; Kubota et al., 2011; Assunção Leme et al., 2013; Moberget et al., 2018; Alnæs et al., 2019), and more recent associations between schizophrenia polygenic score and structure in unaffected individuals (Alnæs et al., 2019; Neilson et al., 2019; Westlye, Alnæs, van der Meer, Kaufmann, & Andreassen, 2019), higher predicted C4A expression was mostly associated with smaller cortical surface area and lower mean cortical thickness (7/11 brain imaging measures, Fig. 2ii). These results, together with our findings on cognitive performance, provide further evidence that some of the common genetic underpinnings of schizophrenia may have similar effects in individuals without mental disorders, in line with dimensional and polygenic risk models (Boyle, Li, & Pritchard, 2017; Purcell et al., 2009; Timpson, Greenwood, Soranzo, Lawson, & Richards, 2018).
In contrast to these results, higher predicted C4A expression was also associated with increased cortical surface area and mean cortical thickness in a subset of brain regions (4/11 brain imaging measures, Fig. 2ii). Among these regions with an increased cortical surface area are the insula and the middle temporal cortices. This is contrary to what is observed in schizophrenia patients where the cortical surface area of these regions is reduced (Assunção Leme et al., 2013; Cobia et al., 2011; Kubota et al., 2011; van Haren et al., 2011). Interestingly, however, a larger cortical surface area has previously been identified in unaffected relatives of schizophrenia patients when compared to non-relative controls (Goghari, Rehm, Carter, & MacDonald, 2007). That study showed that relatives had increased gray matter volume and surface area in the left hemisphere, bilaterally in the parahippocampal gyri, and in the left middle temporal lobe, thereby implicating the cingulate and temporal regions which are known to be associated with higher level cognitive, affective, and memory functions (Goghari et al., 2007). The authors suggested two possible explanations for these observed increases in the gray matter of relatives; (i) abnormal cell migration and deficient pruning, and (ii) a protective or compensatory factor against the development of psychosis or loss of associated functioning (Córdova-Palomera et al., 2018; Goghari et al., 2007). Given the molecular functions of complement C4 in the brain, our results could support their suggestion of altered cell migration and synaptic pruning. Moreover, our mediation analyses also suggest the presence of compensatory factors against C4A-associated cognitive deficits in individuals without mental disorders.
Previous large scale studies investigating the differences in brain imaging measures between schizophrenia patients and healthy controls show prolific effects of the disorder on numerous measures of cortical surface area and thickness (van Erp et al., 2018), as well as subcortical volumes (van Erp et al., 2016). Although these effects are considered small to medium, they are much larger than the effects of C4A expression observed in the present study. Thus, although the changes in brain structure in schizophrenia may be influenced by the level of C4A expression, a large number of genetic and environmental factors likely contribute, as suggested by previous studies (Lee et al., 2016).
Brain imaging measures were previously shown to correlate positively with general cognitive performance in the UK Biobank (Cox et al., 2019). Since we had identified a significant negative effect of C4A expression on cognitive task performance and significant effects on brain imaging measures (predominantly in the negative direction) (Fig. 2), we expected ex ante to observe consistent mediation via the indirect effect (Fig. 2iii, path ab), i.e. that some proportion of the effect of C4A expression on cognitive performance would be accounted for by the effect of C4A expression on brain imaging measures. All of our observations, however, were of inconsistent mediation, i.e. that changes in brain structure, directly or indirectly related to higher C4A expression, may act in a protective or compensatory manner against C4A-associated cognitive deficits. Significant C4A-associated increases in insula surface area were shown to partially mediate the effects of C4A expression on cognitive performance (Fig. 2iii). Specifically, increased insula surface area suppressed the negative effects of C4A expression on episodic memory (Pairs Matching task) and complex processing speed (Symbol Digit Substitution task) by approximately 15% (Fig. 2iii). Despite the significant correlation identified between C4A expression and insula surface area, these mediation results suggest that this relationship is driven by additional components other than C4A expression. Rather, the increase in insula surface area is the result of some undetermined mechanism in response to increased C4A expression. A similar compensatory relationship was identified between C4A expression, cognitive performance, and mean medial orbitofrontal cortical thickness (Fig. 2iii). Increased medial orbitofrontal cortical thickness suppressed the negative effects of C4A expression on episodic memory (Pairs Matching task) by approximately 9% (Fig. 2iii). In this instance, however, predicted C4A expression was negatively associated with mean medial orbitofrontal cortical thickness. Thus, the observed relationship between C4A expression and medial orbitofrontal cortical thickness is likely driven by increased C4A expression, and the observed protective effect is likely driven by another distinct mechanism in order to compensate for the effects of increased C4A expression.
Partial mediation of the effects of C4A expression on cognitive performance, by changes in brain imaging measures, suggests that additional mechanisms play a role in modulating this relationship. Furthermore, given the healthier bias of UK Biobank participants (Fry et al., 2017), further exaggerated by our removal of individuals with mental or neurological disorders, it is tempting to speculate that these participants may share other protective or compensatory factors, in addition to the brain imaging differences identified in this study, which might mask the true effect of C4A expression on cognitive performance. Thus, the true effect would likely be greater in an unbiased population cohort. Future studies should identify additional factors associated with changes in C4A expression and cognitive performance in order to determine other mechanisms that might contribute to their relationship.
A limitation to the current study is that the UK Biobank has an older age distribution in comparison to patients included in most schizophrenia studies, which are commonly conducted on individuals within an age range more closely matching the age of onset of the disorder (18–25 years). As a result, despite controlling for age in our analyses, we cannot exclude a potential effect of aging on the results. Studies in prospective cohorts are required to address this limitation. A second limitation is the reduced sample size for some of the cognitive tasks. Since the identified significant effects of C4A expression of cognitive tasks were small, and predominantly identified for those tasks with the largest sample sizes, these reduced numbers may have resulted in false negatives. Future studies with larger samples for these cognitive tasks are required to determine their true relationship with C4A expression. Finally, the significant effects of C4A expression on cognitive performance and brain morphology identified in this study are very small. By comparison, the effects of brain imaging measures on cognitive performance are magnitudes greater than the effects of C4A expression on cognitive performance (online Supplementary Table S20). This highlights that a large number of additional genetic and environmental factors contribute to these phenotypes.
In conclusion, we observed that higher predicted C4A expression is associated with lower cognitive performance and regional cortical surface area and thickness. Moreover, we provide evidence that the observed changes in cognitive performance, as a result of predicted C4A expression, may be mediated by C4A-associated changes in brain structure. These results demonstrate that C4 locus affects cognition and brain morphology in individuals without mental or neurological disorders.
Acknowledgements
We gratefully acknowledge support from the Research Council of Norway (grant 273291, 248980, 248778 and 248828 for OAA; grant 249795 for LTW), and the South-Eastern Norway Regional Health Authority (grant 2018094 for SD; grant 2020060 for IES; grant 2017-112 for OAA; grant 2019101 for LTW), the European Research Council under the European Union's Horizon 2020 research and innovation program (ERC Starting Grant 802998: BRAINMINT for LTW). This research has been conducted using the UK Biobank Resource (Application number 27412). Dr Andreassen reports personal fees from Lundbeck outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291721000179.
click here to view supplementary material
References
- Alfaro-Almagro, F., Jenkinson, M., Bangerter, N. K., Andersson, J. L. R., Griffanti, L., Douaud, G., … Smith, S. M. (2018). Image processing and quality control for the first 10000 brain imaging datasets from UK Biobank. NeuroImage, 166, 400–424. 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnæs, D., Kaufmann, T., van der Meer, D., Córdova-Palomera, A., Rokicki, J., & Moberget, T., … Karolinska Schizophrenia Project Consortium. (2019). Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry, 76(7), 739–748. 10.1001/jamapsychiatry.2019.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção Leme, I. B., Gadelha, A., Sato, J. R., Ota, V. K., de Mari, J. J., Melaragno, M. I., … Jackowski, A. P. (2013). Is there an association between cortical thickness, age of onset, and duration of illness in schizophrenia? CNS Spectrums, 18(6), 315–321. 10.1017/S1092852913000333. [DOI] [PubMed] [Google Scholar]
- Athanasiu, L., Giddaluru, S., Fernandes, C., Christoforou, A., Reinvang, I., Lundervold, A. J., … Le Hellard, S. (2017). A genetic association study of CSMD1 and CSMD2 with cognitive function. Brain, Behavior, and Immunity, 61, 209–216. 10.1016/j.bbi.2016.11.026. [DOI] [PubMed] [Google Scholar]
- Barch, D. M., & Ceaser, A. (2012). Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Sciences, 16(1), 27–34. 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora, E., Yücel, M., & Pantelis, C. (2010). Cognitive impairment in schizophrenia and affective psychoses: Implications for DSM-V criteria and beyond. Schizophrenia Bulletin, 36(1), 36–42. 10.1093/schbul/sbp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, E. A., Li, Y. I., & Pritchard, J. K. (2017). An expanded view of complex traits: From polygenic to omnigenic. Cell, 169(7), 1177–1186. 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft, C., Freeman, C., Petkova, D., Band, G., Elliott, L. T., Sharp, K., … Marchini, J. (2018). The UK Biobank resource with deep phenotyping and genomic data. Nature, 562(7726), 203–209. 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A, Janeway, J., Travers, P., Walport, M., & Shlomchik, M. J. (2001). The complement system and innate immunity. In Gibbs Sarah (Ed.), Immunobiology: The immune system in health and Disease. New York, USA: Garland Science. https://www.ncbi.nlm.nih.gov/books/NBK27100/. [Google Scholar]
- Cobia, D. J., Csernansky, J. G., & Wang, L. (2011). Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophrenia Research, 133(1–3), 68–76. 10.1016/j.schres.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Córdova-Palomera, A., Kaufmann, T., Bettella, F., Wang, Y., Doan, N. T., van der Meer, D., … Westlye, L. T. (2018). Effects of autozygosity and schizophrenia polygenic risk on cognitive and brain developmental trajectories. European Journal of Human Genetics: EJHG, 26(7), 1049–1059. 10.1038/s41431-018-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, S. R., Ritchie, S. J., Fawns-Ritchie, C., Tucker-Drob, E. M., & Deary, I. J. (2019). Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence, 76, 101376. 10.1016/j.intell.2019.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe, G., Holland, J., Mothersill, D., McCarthy-Jones, S., Cosgrove, D., Harold, D., … Morris, D. W. (2018). Genetically predicted complement component 4A expression: Effects on memory function and middle temporal lobe activation. Psychological Medicine, 48(10), 1608–1615. 10.1017/S0033291717002987. [DOI] [PubMed] [Google Scholar]
- Donohoe, G., Walters, J., Hargreaves, A., Rose, E. J., Morris, D. W., Fahey, C., … Corvin, A. (2013). Neuropsychological effects of the CSMD1 genome-wide associated schizophrenia risk variant rs10503253. Genes, Brain, and Behavior, 12(2), 203–209. 10.1111/gbb.12016. [DOI] [PubMed] [Google Scholar]
- Druart, M., & Le Magueresse, C. (2019). Emerging roles of complement in psychiatric disorders. Frontiers in Psychiatry, 10, 573. 10.3389/fpsyt.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett, A.-K. J., Viechtbauer, W., Dominguez, M.-G., Penn, D. L., van Os, J., & Krabbendam, L. (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neuroscience and Biobehavioral Reviews, 35(3), 573–588. 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2012). Freesurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., … Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fry, A., Littlejohns, T. J., Sudlow, C., Doherty, N., Adamska, L., Sprosen, T., … Allen, N. E. (2017). Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. American Journal of Epidemiology, 186(9), 1026–1034. 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari, V. M., Rehm, K., Carter, C. S., & MacDonald, A. W. (2007). Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cerebral Cortex (New York, N.Y.: 1991), 17(2), 415–424. 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Gorelik, A., Sapir, T., Haffner-Krausz, R., Olender, T., Woodruff, T. M., & Reiner, O. (2017). Developmental activities of the complement pathway in migrating neurons. Nature Communications, 8, 15096. 10.1038/ncomms15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakobyan, S., Boyajyan, A., & Sim, R. B. (2005). Classical pathway complement activity in schizophrenia. Neuroscience Letters, 374(1), 35–37. 10.1016/j.neulet.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Hong, S., Beja-Glasser, V. F., Nfonoyim, B. M., Frouin, A., Li, S., Ramakrishnan, S., … Stevens, B. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science (New York, N.Y.), 352(6286), 712–716. 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, C.-L., Xiang, Y.-T., Wang, Z.-L., Everall, I., Tang, Y., Yang, C., … Jia, F.-J. (2016). Cognitive functioning in individuals at ultra-high risk for psychosis, first-degree relatives of patients with psychosis and patients with first-episode schizophrenia. Schizophrenia Research, 174(1–3), 71–76. 10.1016/j.schres.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Howson, J. M. M., Walker, N. M., Clayton, D., & Todd, J. A., & Type 1 Diabetes Genetics Consortium. (2009). Confirmation of HLA class II independent type 1 diabetes associations in the major histocompatibility complex including HLA-B and HLA-A. Diabetes, Obesity & Metabolism, 11(Suppl. 1), 31–45. 10.1111/j.1463-1326.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitaki, N., Sekar, A., Handsaker, R. E., de Rivera, H., Tooley, K., Morris, D. L., … McCarroll, S. A. (2020). Complement genes contribute sex-biased vulnerability in diverse disorders. Nature, 582, 577–581. 10.1038/s41586-020-2277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, T., van der Meer, D., Doan, N. T., Schwarz, E., Lund, M. J., Agartz, I., … Westlye, L. T. (2019). Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nature Neuroscience, 22(10), 1617–1623. 10.1038/s41593-019-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall, K. M., Bracher-Smith, M., Fitzpatrick, H., Lynham, A., Rees, E., Escott-Price, V., … Kirov, G. (2019). Cognitive performance and functional outcomes of carriers of pathogenic copy number variants: Analysis of the UK Biobank. The British Journal of Psychiatry: The Journal of Mental Science, 214(5), 297–304. 10.1192/bjp.2018.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall, K. M., Rees, E., Escott-Price, V., Einon, M., Thomas, R., Hewitt, J., … Kirov, G. (2017). Cognitive performance among carriers of pathogenic copy number variants: Analysis of 152,000 UK Biobank subjects. Biological Psychiatry, 82(2), 103–110. 10.1016/j.biopsych.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Kubota, M., Miyata, J., Yoshida, H., Hirao, K., Fujiwara, H., Kawada, R., … Murai, T. (2011). Age-related cortical thinning in schizophrenia. Schizophrenia Research, 125(1), 21–29. 10.1016/j.schres.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Laskaris, L., Zalesky, A., Weickert, C. S., Di Biase, M. A., Chana, G., Baune, B. T., … Cropley, V. (2019). Investigation of peripheral complement factors across stages of psychosis. Schizophrenia Research, 204, 30–37. 10.1016/j.schres.2018.11.035. [DOI] [PubMed] [Google Scholar]
- Lee, P. H., Baker, J. T., Holmes, A. J., Jahanshad, N., Ge, T., Jung, J.-Y., … Smoller, J. W. (2016). Partitioning heritability analysis reveals a shared genetic basis of brain anatomy and schizophrenia. Molecular Psychiatry, 21(12), 1680–1689. 10.1038/mp.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, M., Delange, J., Ranjan, R., Meltzer, H. Y., Desnyder, R., Cooremans, W., & Scharpé, S. (1997). Acute phase proteins in schizophrenia, mania and major depression: Modulation by psychotropic drugs. Psychiatry Research, 66(1), 1–11. 10.1016/s0165-1781(96)02915-0. [DOI] [PubMed] [Google Scholar]
- Mayilyan, K. R., Arnold, J. N., Presanis, J. S., Soghoyan, A. F., & Sim, R. B. (2006). Increased complement classical and mannan-binding lectin pathway activities in schizophrenia. Neuroscience Letters, 404(3), 336–341. 10.1016/j.neulet.2006.06.051. [DOI] [PubMed] [Google Scholar]
- Mayilyan, K. R., Dodds, A. W., Boyajyan, A. S., Soghoyan, A. F., & Sim, R. B. (2008a). Complement C4B protein in schizophrenia. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 9(3), 225–230. 10.1080/15622970701227803. [DOI] [PubMed] [Google Scholar]
- Mayilyan, K. R., Weinberger, D. R., & Sim, R. B. (2008b). The complement system in schizophrenia. Drug News & Perspectives, 21(4), 200–210. 10.1358/dnp.2008.21.4.1213349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately, R. I., Giuliano, A. J., Goff, K. P., Faraone, S. V., & Seidman, L. J. (2009). Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology, 23(3), 315–336. 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Miller, K. L., Alfaro-Almagro, F., Bangerter, N. K., Thomas, D. L., Yacoub, E., Xu, J., … Smith, S. M. (2016). Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nature Neuroscience, 19(11), 1523–1536. 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget, T., Doan, N. T., Alnæs, D., Kaufmann, T., Córdova-Palomera, A., Lagerberg, T. V., … Westlye, L. T. (2018). Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: A multisite mega-analysis of 983 patients and 1349 healthy controls. Molecular Psychiatry, 23(6), 1512–1520. 10.1038/mp.2017.106. [DOI] [PubMed] [Google Scholar]
- Neilson, E., Shen, X., Cox, S. R., Clarke, T.-K., Wigmore, E. M., Gibson, J., … Lawrie, S. M. (2019). Impact of polygenic risk for schizophrenia on cortical structure in UK Biobank. Biological Psychiatry, 86(7), 536–544. 10.1016/j.biopsych.2019.04.013. [DOI] [PubMed] [Google Scholar]
- Niculescu, T., Weerth, S., Niculescu, F., Cudrici, C., Rus, V., Raine, C. S., … Rus, H. (2004). Effects of complement C5 on apoptosis in experimental autoimmune encephalomyelitis. Journal of Immunology (Baltimore, Md.: 1950), 172(9), 5702–5706. 10.4049/jimmunol.172.9.5702. [DOI] [PubMed] [Google Scholar]
- Nimgaonkar, V. L., Prasad, K. M., Chowdari, K. V., Severance, E. G., & Yolken, R. H. (2017). The complement system: A gateway to gene-environment interactions in schizophrenia pathogenesis. Molecular Psychiatry, 22(11), 1554–1561. 10.1038/mp.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardiñas, A. F., Holmans, P., Pocklington, A. J., Escott-Price, V., Ripke, S., Carrera, N., … Walters, J. T. R. (2018). Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nature Genetics, 50(3), 381–389. 10.1038/s41588-018-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium, Purcell, S. M., Wray, N. R., Stone, J. L., Visscher, P. M., O'Donovan, M. C., … Sklar, P. (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature, 460(7256), 748–752. 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri, S., Sandor, C., Stahl, E. A., Freudenberg, J., Lee, H.-S., Jia, X., … de Bakker, P. I. W. (2012). Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nature Genetics, 44(3), 291–296. 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, A. F. G., Roalf, D. R., Ruparel, K., Blake, J., Seelaus, K., Villa, L. P., … Satterthwaite, T. D. (2018). Quantitative assessment of structural image quality. NeuroImage, 169, 407–418. 10.1016/j.neuroimage.2017.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. (2011). Genome-wide association study identifies five new schizophrenia loci. Nature Genetics, 43(10), 969–976. 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar, A., Bialas, A. R., de Rivera, H., Davis, A., Hammond, T. R., Kamitaki, N., … McCarroll, S. A.(2016). Schizophrenia risk from complex variation of complement component 4. Nature, 530(7589), 177–183. 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren, C. M., Gracias, J., Watmuff, B., Biag, J. D., Thanos, J. M., Whittredge, P. B., … Perlis, R. H. (2019). Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nature Neuroscience, 22(3), 374–385. 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren, C. M., Sheridan, S. D., Gracias, J., Xuan, D., Fu, T., & Perlis, R. H. (2017). Patient-specific models of microglia-mediated engulfment of synapses and neural progenitors. Molecular Psychiatry, 22(2), 170–177. 10.1038/mp.2016.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J., Levinson, D. F., Duan, J., Sanders, A. R., Zheng, Y., Pe'er, I., … Gejman, P. V. (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature, 460(7256), 753–757. 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeland, O. B., Bahrami, S., Frei, O., Shadrin, A., O'Connell, K., Savage, J., … Andreassen, O. A. (2019). Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. Molecular Psychiatry, 25, 844–853. 10.1038/s41380-018-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby, I. E., Gústafsson, Ó., Doan, N. T., Hibar, D. P., Martin-Brevet, S., & Abdellaoui, A., … 16p11.2 European Consortium, for the ENIGMA-CNV working group. (2018). Dose response of the 16p11.2 distal copy number variant on intracranial volume and basal ganglia. Molecular Psychiatry, 25(3), 584–602. 10.1038/s41380-018-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson, H., Ophoff, R. A., Steinberg, S., Andreassen, O. A., Cichon, S., Rujescu, D., … Collier, D. A. (2009). Common variants conferring risk of schizophrenia. Nature, 460(7256), 744–747. 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan, A. H., Barres, B. A., & Stevens, B. (2012). The complement system: An unexpected role in synaptic pruning during development and disease. Annual Review of Neuroscience, 35, 369–389. 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stokowska, A., Atkins, A. L., Morán, J., Pekny, T., Bulmer, L., Pascoe, M. C., … Pekna, M. (2017). Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain: A Journal of Neurology, 140(2), 353–369. 10.1093/brain/aww314. [DOI] [PubMed] [Google Scholar]
- Tenner, A. J., Stevens, B., & Woodruff, T. M. (2018). New tricks for an ancient system: Physiological and pathological roles of complement in the CNS. Molecular Immunology, 102, 3–13. 10.1016/j.molimm.2018.06.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson, N. J., Greenwood, C. M. T., Soranzo, N., Lawson, D. J., & Richards, J. B. (2018). Genetic architecture: The shape of the genetic contribution to human traits and disease. Nature Reviews. Genetics, 19(2), 110–124. 10.1038/nrg.2017.101. [DOI] [PubMed] [Google Scholar]
- van Beek, J., Nicole, O., Ali, C., Ischenko, A., MacKenzie, E. T., Buisson, A., & Fontaine, M. (2001). Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport, 12(2), 289–293. 10.1097/00001756-200102120-00022. [DOI] [PubMed] [Google Scholar]
- Writing Committee for the ENIGMA-CNV Working Group, van der Meer, D., Sønderby, I. E., Kaufmann, T., Walters, G. B., Abdellaoui, A., … Andreassen, O. A. (2019). Association of copy number variation of the 15q11.2 BP1-BP2 region with cortical and subcortical morphology and cognition. JAMA Psychiatry, 77(4), 420–430. 10.1001/jamapsychiatry.2019.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp, T. G. M., Hibar, D. P., Rasmussen, J. M., Glahn, D. C., Pearlson, G. D., Andreassen, O. A., … Turner, J. A. (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry, 21(4), 547–553. 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp, T. G. M., Walton, E., Hibar, D. P., Schmaal, L., Jiang, W., Glahn, D. C., … Turner, J. A. (2018). Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biological Psychiatry, 84(9), 644–654. 10.1016/j.biopsych.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren, N. E. M., Schnack, H. G., Cahn, W., van den Heuvel, M. P., Lepage, C., Collins, L., … Kahn, R. S. (2011). Changes in cortical thickness during the course of illness in schizophrenia. Archives of General Psychiatry, 68(9), 871–880. 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- Vasek, M. J., Garber, C., Dorsey, D., Durrant, D. M., Bollman, B., Soung, A., … Klein, R. S. (2016). A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature, 534(7608), 538–543. 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, J. T. R., Rujescu, D., Franke, B., Giegling, I., Vásquez, A. A., Hargreaves, A., … Owen, M. J. (2013). The role of the major histocompatibility complex region in cognition and brain structure: A schizophrenia GWAS follow-up. The American Journal of Psychiatry, 170(8), 877–885. 10.1176/appi.ajp.2013.12020226. [DOI] [PubMed] [Google Scholar]
- Wang, C., Yue, H., Hu, Z., Shen, Y., Ma, J., Li, J., … Gu, Y. (2020). Microglia mediate forgetting via complement-dependent synaptic elimination. Science (New York, N.Y.), 367(6478), 688–694. 10.1126/science.aaz2288. [DOI] [PubMed] [Google Scholar]
- Westlye, L. T., Alnæs, D., van der Meer, D., Kaufmann, T., & Andreassen, O. A. (2019). Population-based mapping of polygenic risk for schizophrenia on the human brain: New opportunities to capture the dimensional aspects of severe mental disorders. Biological Psychiatry, 86(7), 499–501. 10.1016/j.biopsych.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Wickham, H. (2009). Ggplot2: Elegant graphics for data analysis. New York, USA: Springer-Verlag. https://www.springer.com/gp/book/9780387981413. [Google Scholar]
- Woo, J. J., Pouget, J. G., Zai, C. C., & Kennedy, J. L. (2019). The complement system in schizophrenia: Where are we now and what's next? Molecular Psychiatry, 25(1), 114–130. 10.1038/s41380-019-0479-0. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Lv, Q., Fan, W., Tang, W., & Yi, Z. (2017). Influence of CFH gene on symptom severity of schizophrenia. Neuropsychiatric Disease and Treatment, 13, 697–706. 10.2147/NDT.S132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291721000179.
click here to view supplementary material