Abstract
Purpose:Sorafenib is recommended for patients with hepatocellular carcinoma refractory to transarterial chemoembolization but with unsatisfactory overall survival and tumor response rate. Previously published studies showed hepatic arterial infusion chemotherapy of oxaliplatin, fluorouracil, and leucovorin was an effective and safe treatment. The aims of this study were to compare the clinical efficacy and safety of oxaliplatin, fluorouracil, and leucovorin-based hepatic arterial infusion chemotherapy with sorafenib in patients with hepatocellular carcinoma refractory to transarterial chemoembolization. Methods: This was a retrospective subgroup analysis of 2 prospective clinical trials, including 114 patients with hepatocellular carcinoma who were confirmed to be transarterial chemoembolization refractoriness. Of these, 55 patients received hepatic arterial infusion chemotherapy of fluorouracil, and leucovorin (FOLFOX-HAIC group, oxaliplatin 85 or 130 mg/m2, leucovorin 400 mg/m2, fluorouracil bolus 400 mg/m2, and 2400 mg/m2 for 23 or 46 h, every 3 weeks), and 59 patients were treated with sorafenib (sorafenib group, 400 mg sorafenib twice daily). Overall survival, progression-free survival, objective response rate, and treatment-related adverse events were compared between the 2 groups. Results: The FOLFOX-HAIC group showed a longer overall survival (17.1 months [95% confidence interval 13.4-20.8] vs 9.1 months [95% confidence interval 7.5-10.6]; hazard ratio 0.35 [95% confidence interval 0.23-0.53]; P < .001), a higher objective response rate (RECIST: 18 [32.7%] vs 1 [1.7%], P < .001), and a longer progression-free survival (7.6 months [95% confidence interval 5.6-9.6] vs 3.9 months [95% confidence interval 2.3-5.4]; hazard ratio 0.49 [95% confidence interval 0.33-0.72]; P < .001) than the sorafenib group. The safety results suggested that both oxaliplatin, fluorouracil, and leucovorin-based hepatic arterial infusion chemotherapy and sorafenib had acceptable treatment-related toxic effects. No significant difference was observed in the overall occurrence of any grade, grade 3/4, or serious adverse events between the 2 groups. Conclusions: Oxaliplatin, fluorouracil, and leucovorin-based hepatic arterial infusion chemotherapy might be a better choice than sorafenib for patients with hepatocellular carcinoma refractory to transarterial chemoembolization.
Keywords: hepatic arterial infusion chemotherapy, hepatocellular carcinoma, oxaliplatin plus fluorouracil and leucovorin, sorafenib, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death globally.1 Surgical resections are considered curative in patients with HCC, but this treatment is not suitable for more than 80% of HCC patients because most patients were diagnosed with unresectable HCC.2,3 A recent Phase III clinical trial (IMbrave150) demonstrated that combination immunotherapy with atezolizumab plus bevacizumab led to significantly improved overall survival and progression-free survival compared with sorafenib in patients with unresectable hepatocellular carcinoma4; therefore, the combined immunotherapy is recommended as first-line systemic therapy for advanced HCC.5,6 Transarterial chemoembolization (TACE) is the standard treatment for patients with intermediate-stage HCC.7,8 However, when repeated, TACE will eventually lose its efficacy, especially in HCC patients with high tumor burden exceeding up to 7 criteria,9 which is called TACE failure/refractoriness according to the Liver Cancer Study Group of Japan criteria.10–13 Compared to continuing TACE, conversion to sorafenib or lenvatinib obviously improved overall survival in patients refractory to TACE,14,15 and sorafenib is recommended for patients with TACE failure/refractoriness by the European Association for the Study of the Liver and American Association for the Study of Liver Diseases.16,17 Based on its non-inferiority to sorafenib in REFLECT trial,18 lenvatinib could be also recommended for HCC patients refractory to TACE. However, the prognosis for these patients receiving sorafenib is poor, with a median time to progression of 2.7 to 3.9 months and a response rate of 6% to 11%.14,19–21
As an alternative therapy to sorafenib, hepatic arterial infusion chemotherapy (HAIC) is recommended for advanced HCC in Japan.10 Besides, the guidelines for HCC of the Japan Society of Hepatology recommend either HAIC or sorafenib for HCC refractory to TACE.10 There were retrospective studies comparing sorafenib with hepatic arterial infusion chemotherapy of cisplatin only, interferon plus 5-fluorouracil, or cisplatin plus 5-fluorouracil.19–23 Most studies demonstrated that sorafenib had better treatment results in HCC with TACE failure/refractoriness compared with HAIC.19,20,23 Conversely, one study showed that HAIC had better overall survival than sorafenib.21 Until now, no prospective randomized controlled trial has not been conducted.
Except for cisplatin-based HAIC, recent studies showed hepatic arterial infusion of oxaliplatin plus fluorouracil and leucovorin (FOLFOX) significantly improved overall survival compared with sorafenib for patients with advanced HCC.24,25 Moreover, our previously published prospective trials showed that HAIC of FOLFOX had better response rates and longer overall survival than TACE, and some patients in the TACE group received FOLFOX-based HAIC or sorafenib after they were considered refractory to transarterial chemoembolization.26,27 Therefore, FOLFOX-based HAIC might be effective for HCC refractory to TACE. Until now, there are no studies comparing the benefits of FOLFOX-based HAIC treatment with sorafenib for HCC refractory to TACE. Herein, we conducted a subgroup analysis of 2 prospective trials published previously to compare the clinical efficacy and safety of FOLFOX-based HAIC with sorafenib in patients with HCC refractory to TACE.
Methods and Materials
Since 2015, our research team conducted studies about FOLFOX-based HAIC at our center in China, and data were prospectively collected on HCC patients undergoing HAIC, TACE, or sorafenib.26–30 This was a retrospective subgroup analysis performed in patients with HCC refractory to TACE from this prospectively collected cohort, and most patients in this study were from these 2 prospective trials.26,27 Ethical approval to report this case series was obtained from the Institutional Review Board of our center, and was conducted in accordance with the Declaration of Helsinki. The study was also registered at http://ClinicalTrials.gov (No. NCT05121571). We have de-identified all patient details in this study. In addition, the reporting of this study conforms to STROBE guidelines.31
Patients
Eligible patients were aged 18 years or older with HCC refractory to TACE according to the Liver Cancer Study Group of Japan criteria: ≥2 consecutive ineffective responses of treated tumors (viable lesions >50%) or ≥2 consecutive progressions in the tumor (including an addition in the number of tumors compared with the previous TACE procedure), even after changing the chemotherapeutic agents or location of the feeding artery, on tumor response evaluation computed tomography (CT) or magnetic resonance imaging (MRI) after 1 to 3 months following adequately performed selective TACE procedure. Further criteria for the classification of TACE failure include the appearance of vascular invasion and extrahepatic spread, and persistent elevation of α-fetoprotein (AFP) within 2 months after TACE exceeding 20% relative to the baseline (before TACE) was considered nonresponse.10–12
Other eligibility criteria were as follows: no extrahepatic metastasis, Child-Pugh class A, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1, not suitable for curative surgery or local ablation, no previous antitumor treatment for HCC except for TACE, at least one measurable lesion based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,32 at least a cycle of HAIC or 3 weeks of sorafenib, and adequate organ function based on the prior study28: leukocyte count ≥3.0 × 109/L, absolute neutrophils count ≥1.5 × 109/L, platelet cell count ≥75 × 109/L, transaminase ≤5 times the upper limit of the normal range.
The exclusion criteria consisted of: hepatic decompensation based on the European Association for the Study of the Liver guidelines,33 deficient blood supply of tumor indicated from CT or MRI arterial phase; HIV infection; pregnancy or breastfeeding; a second malignancy; patients who were treated with both HAIC and sorafenib during the follow-up period; lost to follow-up; and lack of the image prior to initiation of the sorafenib or HAIC.
Treatments
When HCC refractory to TACE was confirmed, a multidisciplinary treatment team including radiologists, surgeons, hepatologists, and oncologists would evaluate the patient's condition and determine whether HAIC or sorafenib is appropriate. Then, every patient was informed of the efficacy and safety of HAIC and sorafenib. Finally, the treatment option of either HAIC or sorafenib was made by the patient. Most patients rejected sorafenib because its high cost. The written informed consents were signed at the first hospitalization and kept in the medical record.
Sorafenib cohort
All patients were treated with 400 mg sorafenib twice daily at first. Sorafenib interruptions and dose reductions were based on the previous study.34 If patients can't tolerate the lowest dose, sorafenib would be discontinued.
HAIC cohort
In the FOLFOX-HAIC group, HAIC was performed on days 1 to 2 as previous studies reported.26–28 A catheter was inserted into the truncus coeliacus or superior mesenteric artery for arteriography. Then a microcatheter was ultra-selectively inserted and located in the main feeding hepatic artery depending on the arterial supply of the tumor. The FOLFOX regimen was infused via the microcatheter: oxaliplatin 85 or 130 mg/m2 from hour 0 to 2 on day 1; leucovorin 400 mg/m2 from hour 2 to 3 on day 1; 5-fluorouracil 400 mg/m2 bolus at hour 3; and 2400 mg/m2 over 23 or 46 h. After the regimen was completed, the catheter was removed.
Follow-Up and Assessments
Sorafenib or HAIC was kept on until one of the following situations occurred: tumor progression, intolerable toxicity, the need of surgery owing to downstaging, or at the patient's request. Follow-up and assessments were the same as our previously published trial.28 Before the treatment was discontinued, blood examination and safety assessment were conducted every 3 weeks. Additionally, upper abdomen-enhanced CT or MRI and chest-enhanced CT were performed every 6 weeks. Tumor response assessments were retrospectively evaluated by 2 independent and experienced radiologists who do not know treatment groups based on RECIST 1.1. If there was a controversy in tumor assessments, the final judgment was made by another more experienced radiologist.
Overall survival was calculated from the date of start of treatment to death from any cause or date of the last follow-up. Progression-free survival was calculated from the date of start of treatment to progression by RECIST 1.1 criteria or death from any cause, whichever occurred first. The objective response rate was the percentage of patients who achieved complete response or partial response based on RECIST version 1.1. Adverse events were assessed by the National Cancer Institute Common Terminology Criteria for adverse events version 4.03. Besides, the tumor response rate was also evaluated based on the modified RECIST (mRECIST) guidelines.35
Statistical Analyses
Student t tests or χ2 tests were used to compare the results. Kaplan-Meier method and log-rank tests were used to compare survival outcomes. A multivariable Cox proportional hazards model was used to analyze factors with P < .10 using univariate analysis. P < .05 was considered significant, and SAS version 9.0 (SAS Institute) was used to perform analyses.
Results
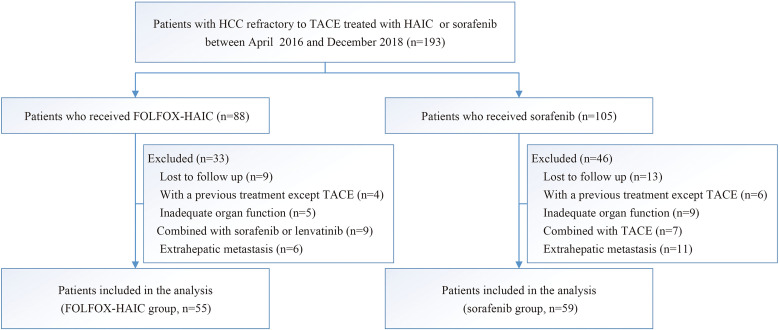
Between April 21, 2016, and December 18, 2018, 193 consecutive patients who were confirmed to be TACE failure/refractoriness were treated using either HAIC or sorafenib, and 114 patients were up to the criteria for inclusion in this study: 55 received FOLFOX-based HAIC, and 59 received sorafenib (Figure 1). The follow-up was finished on May 1, 2021. There was no difference in the baseline characteristics between groups (Table 1). Patients consisted of 106 males and 8 females, and mean age (standard deviation [SD]) was 49.1 years (11.5). Most patients had hepatitis B virus infection (82.5%), multiple lesions (78.1%), and absence of portal vein tumor thrombus (69.3%). The mean and median size of the maximum tumor measured by RECIST criteria was 10.3 cm (SD 3.6) and 10.3 cm (interquartile range [IQR] 8.2-12.9). Treatment administration after disease progression is listed in Table 1. After the termination of the study treatment due to disease progression, 17 patients received subsequent treatments, including Sorafenib (7), Lenvatinib (6), PD-1 inhibitor treatment (1), and Radiology (3) in the FOLFOX-HAIC group. In the Sorafenib group, 25 patients received subsequent treatments after disease progression, including FOLFOX-HAIC (3), Lenvatinib (7), Regorafenib (8), PD-1 inhibitor treatment (5), and Radiology (2). More patients in the FOLFOX-HAIC group than those in the Sorafenib group received Sorafenib (7 vs 0, P < .05). Instead, more patients in the Sorafenib group received Regorafenib (8 vs 0, P < .05) than those in the FOLFOX-HAIC group. In summary, there was no significant difference in the subsequent treatments between these 2 groups.
Figure 1.
Trial profile. Abbreviations: FOLFOX-HAIC group, hepatic arterial infusion chemotherapy of oxaliplatin, 5-fluorouracil, and leucovorin group; HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma; Sorafenib group, sorafenib monotherapy group; TACE, transarterial chemoembolization.
Table 1.
Demographics and Baseline Characteristics.a
| FOLFOX-HAIC group (n = 55) | Sorafenib group (n = 59) | P | |
|---|---|---|---|
| Age | .19 | ||
| ≤50 | 30 | 25 | |
| >50 | 25 | 34 | |
| Sex | .48 | ||
| Male | 50 | 56 | |
| Female | 5 | 3 | |
| ECOG performance status | .20 | ||
| 0 | 16 | 24 | |
| 1 | 39 | 35 | |
| HBV infection | .51 | ||
| Yes | 44 | 50 | |
| No | 11 | 9 | |
| Maximum tumor diameter, cm | .19 | ||
| ≤10 | 23 | 32 | |
| >10 | 32 | 27 | |
| Tumor number | .98 | ||
| Single | 12 | 13 | |
| Multiple | 43 | 46 | |
| Portal vein tumor thrombus | .65 | ||
| Absence | 37 | 42 | |
| Presence | 18 | 17 | |
| BCLC stage | .51 | ||
| B | 35 | 41 | |
| C | 20 | 18 | |
| AFP, ng/mL | .93 | ||
| ≤1000 | 21 | 23 | |
| >1000 | 34 | 36 | |
| Child-Pugh score | .67 | ||
| 5 | 48 | 53 | |
| 6 | 7 | 6 | |
| mALBI grade | .52 | ||
| 1 | 32 | 34 | |
| 2a | 15 | 20 | |
| 2b | 8 | 5 | |
| Therapy after progression | |||
| Sorafenib | 7 | 0 | .005 |
| FOLFOX-HAIC | 0 | 3 | .24 |
| Lenvatinib | 6 | 7 | .87 |
| Regorafenib | 0 | 8 | .006 |
| PD-1 inhibitor treatment | 1 | 5 | .21 |
| Radiotherapy | 3 | 2 | .67 |
Abbreviations: FOLFOX-HAIC group, hepatic arterial infusion chemotherapy of oxaliplatin, 5-fluorouracil and leucovorin group; Sorafenib group, Sorafenib monotherapy group; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; BCLC, Barcelona Clinic Liver Cancer staging; AFP, alpha-fetoprotein; mALBI grade, modified albumin-bilirubin grade.
Data are expressed as n. P value was calculated by Student t tests or χ2 tests.
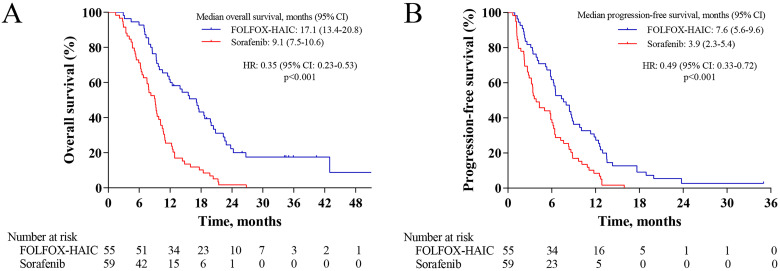
At the time of analysis, 103 patients had died (44 patients in the FOLFOX-HAIC group and 59 patients in the sorafenib group). The median overall survival in the FOLFOX-HAIC group was 17.1 months (95% confidence interval [CI] 13.4-20.8) compared with 9.1 months (95% CI 7.5-10.6) in the sorafenib group (hazard ratio [HR] 0.35 [95% CI 0.23-0.53]; P < .001; Figure 2A). The overall survival rates at 6, 12, and 18 months were 92.7%, 61.8%, and 41.8% in the FOLFOX-HAIC group and 71.2%, 25.4%, and 10.2% in the sorafenib group, respectively. The results of univariable and multivariable analysis of overall survival are listed in Table 2. These factors with a P < .1 were selected for multivariate analysis, including type of treatment, absence or presence of portal vein tumor thrombus, and AFP level. Multivariable analysis showed that independent risk factors for survival were treatment allocation (FOLFOX-HAIC vs sorafenib, HR = 0.29; 95% CI, 0.19-0.44; P < .001), and absence or presence of portal vein tumor thrombus (HR = 0.49; 95% CI, 0.31-0.77; P = .002).
Figure 2.
Kaplan-Meier curves for overall survival and progression-free survival. (A) Overall survival; (B) progression-free survival. Abbreviations: CI, confidence interval; FOLFOX-HAIC group, hepatic arterial infusion chemotherapy of oxaliplatin, 5-fluorouracil and leucovorin group; HR, hazard ratio; Sorafenib group, sorafenib monotherapy group.
Table 2.
Univariate and Multivariate Analysis of Overall Survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Overall survival, months | P a | HR (95% CI) | P b | |
| Group (FOLFOX-HAIC/Sorafenib) | 17.1 vs 9.07 | <.001 | 0.29 (0.19-0.44) | <.001 |
| Age, years (≤50/>50) | 9.4 vs 10.63 | .88 | ||
| Sex (male/female) | 10.17 vs 10.63 | .56 | ||
| ECOG-PS (0/1) | 11.1 vs 9.47 | .16 | ||
| HBV infection (yes/no) | 10.63 vs 7.83 | .36 | ||
| Tumor size, cm (≤10/>10) | 10.93 vs 9.7 | .67 | ||
| Tumor number (single/multiple) | 7.83 vs 10.93 | .26 | ||
| PVTT (absence/presence) | 12.4 vs 8.53 | .02 | 0.49 (0.31-0.77) | .002 |
| AFP, ng/mL (≤1000/>1000) | 10.97 vs 9.4 | .09 | 0.71 (0.47-1.07) | .1 |
| mALBI (1/2a/2b) | 11.1 vs 9.7 vs 7.83 | .27 | ||
| Child-Pugh score (5 vs 6) | 10.73 vs 9.13 | .12 | ||
Abbreviations: FOLFOX, oxaliplatin, 5-fluorouracil and leucovorin; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; PVTT, portal vein tumor thrombus; AFP, alpha-fetoprotein; mALBI, modified albumin-bilirubin grade.
P value was calculated with a 2-sided log-rank test. Any factors that were statistically significant at P less than 10% in the univariate analysis were candidates for entry into a multivariable Cox analysis.
P value was calculated by multivariable Cox proportional-hazards analysis.
The median progression-free survival in the FOLFOX-HAIC group was 7.6 months (95% CI 5.6-9.6) compared with 3.9 months (95% CI 2.3-5.4) in the sorafenib group (HR 0.49 [95% CI 0.33-0.72]; P < .001; Figure 2B). The objective response rate was significantly greater in the FOLFOX-HAIC group than in the sorafenib group (40% vs 3.4% by mRECIST criteria, P < .001; 32.7% vs 1.7% by RECIST criteria, P < .001; Table 3). CT scans of 5 representative patients are shown in Supplement materials.
Table 3.
Summary of Best Tumor Response.a
| Response (RECIST) | Response (mRECIST) | |||||
|---|---|---|---|---|---|---|
| FOLFOX-HAIC group (%) | Sorafenib group (%) | P | FOLFOX-HAIC group (%) | Sorafenib group (%) | P | |
| CR | 0 (0) | 0 (0) | - | 3 (5.5%) | 0 (0) | .11 |
| PR | 18 (32.7%) | 1 (1.7%) | <.001 | 19 (34.5%) | 2 (3.4%) | <.001 |
| SD | 26 (47.3%) | 36 (61.0%) | .14 | 22 (40.0%) | 35 (59.3%) | .04 |
| PD | 11 (20.0%) | 22 (37.3%) | .04 | 11 (20.0%) | 22 (37.3%) | .04 |
| ORR | 18 (32.7%) | 1 (1.7%) | <.001 | 22 (40.0%) | 2 (3.4%) | <.001 |
Abbreviations: FOLFOX-HAIC group, hepatic arterial infusion chemotherapy of oxaliplatin, 5-fluorouracil and leucovorin group; Sorafenib group, sorafenib monotherapy group; mRECIST, modified Response Evaluation Criteria in Solid Tumors; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate.
Data are expressed as n (%). ORR was the proportion of patients with complete response or partial response. Statistical significance was assessed with the χ2 test.
Treatment-related adverse events with a high incidence rate (≥10%) were shown in Table 4. There was no difference in the overall occurrence of treatment-related adverse events between the 2 groups (any grade: 52 [94.5%] vs 51 [86.4%], P = .14; grade 3/4: 18 [32.7%] vs 23 [39.0%], P = .49). The frequencies of all-grade hypertension, hand–foot skin reaction, alopecia, and rash were significantly higher in the sorafenib group than in the FOLFOX-HAIC group, whereas the frequencies of all-grade nausea, vomiting, thrombocytopenia, sensory neuropathy, hypoalbuminemia, and abdominal pain were significantly higher in the FOLFOX-HAIC group. The grade 3 to 4 vomiting was more frequent in the FOLFOX-HAIC group (P = .03), while the grade 3 to 4 hand–foot skin reaction was more frequent in the sorafenib group (P = .003). Serious adverse events occurred in 6 (10.9%) of 55 patients who received FOLFOX-based HAIC, and 8 (13.6%) of 59 patients who received sorafenib (P = .67). Besides, 3 patients with thrombosis or dislocation of the catheter tip in the FOLFOX-HAIC group were recatheterized, and no severe complications associated with the intra-arterial technique were observed.
Table 4.
Treatment-Related Adverse Events.a,b
| Adverse event | FOLFOX-HAIC group (n = 55) | Sorafenib group (n = 59) | P value | |||
|---|---|---|---|---|---|---|
| Any grade | Grade 3-4 | Any grade | Grade 3-4 | Any grade | Grade 3-4 | |
| Total | 52 | 18 | 51 | 23 | .14 | .49 |
| Blood/bone marrow suppression | ||||||
| Neutropenia | 15 | 5 | 9 | 1 | .12 | .11 |
| Thrombocytopenia | 20 | 3 | 8 | 0 | .005 | .11 |
| Anemia | 22 | 3 | 18 | 2 | .29 | .67 |
| Cardiovascular system | ||||||
| Hypertension | 2 | 0 | 13 | 2 | .004 | .5 |
| Edema | 7 | 1 | 4 | 0 | .28 | .48 |
| Constitutional symptoms | ||||||
| Fatigue | 30 | 1 | 23 | 4 | .1 | .37 |
| Weight loss | 13 | 0 | 19 | 2 | .31 | .5 |
| Dermatologic events | ||||||
| Hand–foot skin reaction | 1 | 0 | 27 | 9 | <.001 | .003 |
| Alopecia | 2 | 0 | 14 | 0 | .002 | - |
| Rash | 2 | 0 | 16 | 2 | .001 | .5 |
| Gastrointestinal events | ||||||
| Nausea | 34 | 5 | 14 | 1 | <.001 | .11 |
| Vomiting | 32 | 7 | 12 | 1 | <.001 | .03 |
| Diarrhea | 20 | 1 | 24 | 3 | .64 | .62 |
| Abdominal pain | 22 | 2 | 2 | 0 | <.001 | .23 |
| Neurotoxicity | ||||||
| Sensory neuropathy | 21 | 0 | 0 | 0 | <.001 | - |
| Hepatic function | ||||||
| Elevated ALT | 33 | 4 | 40 | 6 | .82 | .74 |
| Elevated AST | 38 | 3 | 43 | 7 | .66 | .32 |
| Hyperbilirubinemia | 31 | 3 | 25 | 2 | .14 | .67 |
| Hypoalbuminemia | 40 | 2 | 20 | 0 | <.001 | .23 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; FOLFOX-HAIC group, hepatic arterial infusion chemotherapy of oxaliplatin, 5-fluorouracil and leucovorin group; Sorafenib group, sorafenib monotherapy group.
Data are expressed as n. The safety population comprised all randomized patients who received at least one dose of study treatment. P value was calculated by a 2-sided χ2 test.
Listed are adverse events, as defined by the National Cancer Institute Common Terminology Criteria (version 4.03), that occurred in at least 10% of patients in either study group.
Discussion
Sorafenib is recommended for patients with TACE failure/refractoriness,8,16,17 but with unsatisfactory tumor response rate and time to progression. Previously published prospective trials showed that FOLFOX-based HAIC had better results than TACE,26 but no study has compared FOLFOX-based HAIC with sorafenib in patients with HCC refractory to TACE. This retrospective subgroup analysis of 2 prospective trials26,27 showed that patients in the FOLFOX-HAIC group had a significantly longer median progression-free survival (7.6 vs 3.9 months), a longer overall survival (17.1 vs 9.1 months), and higher radiologic objective response rate (32.7% vs 1.7% per RECIST 1.1 criteria) than patients in the sorafenib group with TACE failure. Treatment allocation as well as absence or presence of portal vein tumor thrombus were independent factors for overall survival. In addition, both FOLFOX-based HAIC and sorafenib had acceptable treatment-related toxic effects.
The survival benefit of FOLFOX-based HAIC versus sorafenib reported in HCC patients refractory to TACE may be due to the powerful antitumor activity of FOLFOX-HAIC. First, chemotherapy regimens in prior studies comparing HAIC with sorafenib for HCC refractory to TACE were cisplatin or cisplatin plus 5-fluorouracil, from which sorafenib had better treatment results.19,20,22,23 However, chemotherapy regimens in our study were oxaliplatin plus fluorouracil and leucovorin, and oxaliplatin has distinct biochemical, pharmacological, and cytotoxic advantages compared with cisplatin.36–38 FOLFOX regimen may be more sensitive to HCC on the basis of EACH study showing the modest antitumor activity.39 Second, to avoid the confounding impact of deaths unrelated to tumor progression, we selected patients with Child-Pugh class A, adequate organ function, and ECOG PS of 0-1, so that patients could tolerate longer periods of treatment cycles. Finally, different from an implanted port catheter system, repetitive indwelling catheter and digital subtraction angiography before each HAIC can confirm the chemotherapeutic drugs in the tumor and avoid drug flow to other organs, which may help to enhance antitumor effects or reduce treatment-related adverse events.
In this subgroup analysis, survival rate in the sorafenib group was lower than that reported in previous trials.19–23 The explanation of these differences was that the patient population enrolled in our study might be considered to present with more advanced disease associated with worse prognosis: mean tumor size larger than 10 cm, and AFP level of 61.4% of patients was higher than 1000 ng/mL. Moreover, the objective response rate in our study was lower than that reported by our previous study (32.7% vs 52.6% by RECIST criteria).26 The reason may be that patients in the previous study were newly diagnosed patients with no previous treatment, while patients in our study had received TACE previously, the chemotherapy regimens of which included platinum, so some HCCs might be resistant to FOLFOX. Other trials also showed that FOLFOX-based HAIC in combination with systemic therapy was an effective and safe treatment option for advanced HCC.28,30 However, whether combined treatments are better than HAIC monotherapy for HCC refractory to TACE needs further research.
Recently, other systemic treatments, including atezolizumab plus bevacizumab, sintilimab plus bevacizumab, yielded a superior tumor response rate than sorafenib.4,40 Several latest practice guidelines and expert consensus statements recommend these systemic treatments for patients with HCC refractory to TACE or exceeding the up to 7 criteria, in spite of the absence of high-level evidence.41–43 However, for high-risk (tumor invasion of the main trunk of the portal vein [Vp4] or tumor involvement ≥50% of liver) patients receiving atezolizumab plus bevacizumab, the prognosis was extremely poor, with a median overall survival of 7.6 months (95% CI 6.6-12.8).44 In contrast, FOLFOX-based HAIC displayed strong anti-tumor effect for HCC patients with a high intrahepatic tumor burden, including those with high-risk status.26,27 Head-to-head comparisons of FOLFOX-based HAIC versus these systemic therapies in such patient population will be needed.
In addition, the treatment-related adverse events were consistent with those observed in previous studies, which seemed to be acceptable and manageable.26,45 No significant difference was observed in the overall occurrence of any grade, grade 3/4, or serious adverse events between these 2 groups. Likely due to the cytotoxic agents oxaliplatin and fluorouracil, the adverse events that were more common in the FOLFOX-HAIC group (eg, nausea, vomiting, hypoalbuminemia, sensory neuropathy, abdominal pain and thrombocytopenia) were mainly mild to moderate, and easily identified, prevented, and treated. The adverse events that were more common in the sorafenib group (eg, hypertension, hand–foot skin reaction, alopecia and rash) were sorafenib-specific. In addition, there were no severe complications associated with the intra-arterial technique in the FOLFOX-HAIC group.
The present study has some limitations. First, this was a small sample, single-center, retrospective subgroup analysis, even though most patients were identified from 2 prospective trials and the baseline demographics were well balanced. However, it could easily lead to a certain bias in the results because the choice of treatment modality was based on the general status of the patients and left mainly to the patients. Second, this study was performed only in China, where HCC patients with a high intrahepatic tumor burden and higher AFP were common, for which patient selection might be one of the reasons for the limited effect of Sorafenib in this study. These findings warrant further validation in a larger patient population with hepatitis C virus infection and a lower tumor burden. Finally, only patients with Child-Pugh class A and ECOG PS of 0 to 1 were included in this study. Its generalizability to patients with more advanced cirrhosis and poorer health states remains further study.
Conclusion
In conclusion, this study showed that patients who received FOLFOX-based HAIC had significantly better progression-free survival, overall survival, and objective response rate than patients who received sorafenib. The safety results suggest that FOLFOX-based HAIC had acceptable treatment-related toxic effects. Thus, FOLFOX-based HAIC might be a better choice than sorafenib for patients with HCC refractory to TACE. A multicenter, randomized, prospective trial should be conducted to demonstrate the efficacy of FOLFOX-based HAIC in patients with HCC refractory to TACE.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338221117389 for Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Sorafenib for Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization: Retrospective Subgroup Analysis of 2 Prospective Trials by YeXing Huang, LiHong Zhang, MinKe He, ZhiCheng Lai, XiaoYun Bu, DongSheng Wen, QiJiong Li, Li Xu, Wei Wei, YaoJun Zhang, ZhongGuo Zhou, MinShan Chen, RongPing Guo, Ming Shi and Anna Kan in Technology in Cancer Research & Treatment
Acknowledgments
The authors thank all the patients and their family members for their permission in this study. The authors also thank statistician Ying Guo for the assistance of biomedical informatics analysis.
Abbreviations
- AFP
alpha-fetoprotein
- CI
confidence interval
- CT
computed tomography
- DCR
disease control rate
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- FOLFOX
Oxaliplatin, Fluorouracil, and Leucovorin
- HAIC
hepatic arterial infusion chemotherapy
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- MRI
magnetic resonance imaging
- ORR
objective response rate
- OS
overall survival
- PFS
progression-free survival
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
standard deviation
- TACE
transarterial chemoembolization.
Footnotes
Authors’ Note: YeXing Huang, LiHong Zhang, MinKe He, and ZhiCheng Lai contributed equally to this work. This study was registered at http://ClinicalTrials. gov (No. NCT05121571). Informed consent was obtained from all individual participants included in the study at their first hospitalization. This retrospective subgroup analysis of 2 prospective trials was performed in line with the principles of the Declaration of Helsinki. Ethical approval was granted by the institutional review board of Sun Yat-sen University Cancer Center in China (B2021-383-01). Written informed consent was obtained from the patients for their anonymized information to be published in this article at their first hospitalization.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the China Postdoctoral Science Foundation, National Natural Science Foundation of China, Development Planned Project in Key Areas of Guangdong Province, Key Technologies Research and Development Program, (grant number 2021TQ0383, No.82072610, No.82102985, 2019B110233002, 2017YFA0505803).
ORCID iDs: QiJiong Li https://orcid.org/0000-0002-3149-1645
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J M, Kelley R K, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. [DOI] [PubMed] [Google Scholar]
- 3.Yang J D, Hainaut P, Gores G J, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn R S, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894‐1905. [DOI] [PubMed] [Google Scholar]
- 5.Vogel A, Martinelli E, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org; ESMO Guidelines Committee, et al Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol. 2021. 32(6): 801‐805. [DOI] [PubMed] [Google Scholar]
- 6.Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541‐565. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver . EASL Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1): 182‐236. [DOI] [PubMed] [Google Scholar]
- 8.Marrero J A, Kulik L M, Sirlin C B, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723‐750. [DOI] [PubMed] [Google Scholar]
- 9.Raoul J L, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer. 2014;3(2):119‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Matsui O, Izumi N, et al. JSH Consensus-Based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3-4):458‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339‐364. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22‐31. [DOI] [PubMed] [Google Scholar]
- 13.Zhong B Y, Wang W S, Zhang S, et al. Re-evaluating transarterial chemoembolization failure/refractoriness: a survey by Chinese College of Interventionalists. J Clin Transl Hepatol. 2021;9(4):521‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87(6):330‐341. [DOI] [PubMed] [Google Scholar]
- 16.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238‐iv255. [DOI] [PubMed] [Google Scholar]
- 17.Omata M, Cheng A L, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Finn R S, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163‐1173. [DOI] [PubMed] [Google Scholar]
- 19.Hatooka M, Kawaoka T, Aikata H, et al. Comparison of outcome of hepatic arterial infusion chemotherapy and sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Anticancer Res. 2016;36(7):3523‐3529. [PubMed] [Google Scholar]
- 20.Ikeda M, Mitsunaga S, Shimizu S, et al. Efficacy of sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. J Gastroenterol. 2014;49(5):932‐940. [DOI] [PubMed] [Google Scholar]
- 21.Moriya K, Namisaki T, Sato S, et al. Efficacy of bi-monthly hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. J Gastrointest Oncol. 2018;9(4):741‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodama K, Kawaoka T, Aikata H, et al. Comparison of clinical outcome of hepatic arterial infusion chemotherapy and sorafenib for advanced hepatocellular carcinoma according to macrovascular invasion and transcatheter arterial chemoembolization refractory status. J Gastroenterol Hepatol. 2018;33(10):1780‐1786. [DOI] [PubMed] [Google Scholar]
- 23.Shiozawa K, Watanabe M, Ikehara T, et al. [Efficacy of sorafenib versus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma refractory to transcatheter arterial chemoembolization]. Gan To Kagaku Ryoho. 2015;42(8):953‐956. [PubMed] [Google Scholar]
- 24.Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. Sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 25.Lyu N, Zhao M. Hepatic arterial infusion chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase 3 trial (the FOHAIC-1 study). J Clin Oncol. 2021;39(15):4007‐4007. [DOI] [PubMed] [Google Scholar]
- 26.He M K, Le Y, Li Q J, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer. 2017;36(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q J, He M K, Chen H W, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol. 2021:JCO2100608. [DOI] [PubMed] [Google Scholar]
- 28.He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7)953‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He M K, Zou R H, Li Q J, et al. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Intervent Radiol. 2018;41(5):734‐743. [DOI] [PubMed] [Google Scholar]
- 30.He M-K, Liang R-B, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Elm E, Altman D G, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐577. [DOI] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 33.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver . EASL Clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018; 69(2): 406‐460. [DOI] [PubMed] [Google Scholar]
- 34.Llovet J M, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378‐390. [DOI] [PubMed] [Google Scholar]
- 35.Lencioni R, Llovet J M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52‐60. [DOI] [PubMed] [Google Scholar]
- 36.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482‐491. [DOI] [PubMed] [Google Scholar]
- 37.Bruno P M, Liu Y, Park G Y, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. 2017;23(4):461‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzodic R, Gomez-Abuin G, Rougier P, et al. Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: comparative results with cisplatin using a rabbit VX2 tumor model. Anticancer Drugs. 2004;15(6):647‐650. [DOI] [PubMed] [Google Scholar]
- 39.Qin S, Bai Y, Lim H Y, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501‐3508. [DOI] [PubMed] [Google Scholar]
- 40.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22(7):977‐990. [DOI] [PubMed] [Google Scholar]
- 41.Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan society of hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109‐1113. [DOI] [PubMed] [Google Scholar]
- 42.Kudo M, Han K H, Ye S L, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9(3):245‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llovet JM, Villanueva A, Marrero JA, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73(Suppl 1):158‐191. [DOI] [PubMed] [Google Scholar]
- 44.Finn R S, Qin S, Ikeda M, et al. Abstract CT009: IMbrave150: updated efficacy and safety by risk status in patients (pts) receiving atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first-line treatment for unresectable hepatocellular carcinoma (HCC). Cancer Res. 2021;81(13 Supplement):CT009‐CT009. [Google Scholar]
- 45.Cheng A L, Kang Y K, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25‐34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338221117389 for Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Sorafenib for Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization: Retrospective Subgroup Analysis of 2 Prospective Trials by YeXing Huang, LiHong Zhang, MinKe He, ZhiCheng Lai, XiaoYun Bu, DongSheng Wen, QiJiong Li, Li Xu, Wei Wei, YaoJun Zhang, ZhongGuo Zhou, MinShan Chen, RongPing Guo, Ming Shi and Anna Kan in Technology in Cancer Research & Treatment