We measured metabolic rates of adult native westslope cutthroat trout and non-native rainbow trout and the critical thermal maximum of adult westslope cutthroat trout. The results will inform and parametrize models used in the recovery planning of westslope cutthroat trout.
Keywords: westslope cutthroat trout, respirometry, metabolic rate, critical thermal maximum, Aerobic scope
Abstract
Global warming is changing the thermal habitat of cold-water freshwater fishes, which can lead to decreased fitness and survival and cause shifts in species distributions. The Alberta population of westslope cutthroat trout (Oncorhynchus clarkii lewisi) is listed as ‘Threatened’ under the Canadian Species at Risk Act. The major threats to the species are the alteration in habitat and water flow, competition and hybridization with non-native trout species and climate change. Here, we conducted (i) intermittent-flow respirometry experiments with adult native westslope cutthroat trout and non-native rainbow trout (Oncorhynchus mykiss) and (ii) critical thermal maximum experiments (CTmax) with adult westslope cutthroat trout to obtain valuable input data for species distribution models. For both species, standard metabolic rate (SMR) was lower at 10°C compared to 15°C and westslope cutthroat trout had higher SMR than rainbow trout. Although there were inter-specific differences in SMR, forced aerobic scope (using a standardized chase protocol) was different at 10°C, but no significant differences were observed at 15°C because of relative smaller differences in maximum metabolic rate between the species. CTmax of westslope cutthroat trout acclimated to 10°C was 27.0 ± 0.8°C and agitation temperature was 25.2 ± 1.0°C. The results from this study will inform and parametrize cumulative effects assessments and bioenergetics habitat modelling for the recovery planning of the species.
Introduction
Regarding warming temperatures and climate change, the biogeographical principle of ‘adapting, migrating or going extinct’ is highly relevant for cold-water fishes (Munday et al., 2017). Populations can adapt to changing temperature regimes through evolution or phenotypic plasticity and/or by migrating to more suitable thermal habitat. The effects of temperature on the metabolic rates, aerobic scope and upper critical temperature limits of cold-water fishes are of particular interest and therefore widely studied (Imholt et al., 2011; Durhack, 2020; Morrison et al., 2020; Macnaughton et al., 2021) as they represent valuable input parameters for various modelling approaches including, e.g. bioenergetics habitat models (Naman et al., 2020), species distribution models (Pandit et al., 2017), minimum viable population analyses (van der Lee and Koops, 2020) and cumulative effects assessments (DFO, 2019a). In particular, aerobic scope, which is defined as the scope of aerobic metabolic rates above the maintenance levels scope (Fry, 1947) and estimated as the difference between the maximum metabolic rate and the standard metabolic rate (SMR), provides insight into a fish’s upper capacity to supply oxygen for life processes such as reproduction, growth and activity in addition to its minimum level necessary to maintain homeostasis (Nelson, 2016). The aerobic scope generally follows a curve, peaking at an optimal temperature (Topt) before decreasing with increasing water temperature (Fry, 1947) where eurythermal species that are thermal generalists have a high aerobic scope over a broad range of temperatures whereas stenothermal species that are thermal specialists have an aerobic scope optimum over a narrow range of temperatures (Durhack et al., 2021; Macnaughton et al., 2021). Quantifying how a species physiologically reacts at different temperatures helps us understand its thermal thresholds and assists with delineating important thermal critical habitats.
Upper thermal limits in fish can be estimated using non-lethal, acute temperature experiments (e.g. Critical Thermal Maximum (CTmax) and agitation temperature (Tag)). CTmax is a useful, repeatable estimate of the sublethal upper temperature limit of a species and defined as the temperature when an individual looses equilibrium and is unable to maintain an upright position in the experimental chamber (Becker and Genoway, 1979). CTmax is likely several degrees higher than the temperature a species can tolerate over prolonged time periods. Therefore, the temperature where a fish is showing an avoidance response and is trying to escape from increasing water temperature (McDonnell and Chapman, 2015) is thought to be more ecologically relevant. The temperature, which triggers an avoidance behaviour at which the fish abandon their habitat to seek shelter in colder habitat, e.g. tributaries or thermal refugia, is defined as the agitation temperature (Tag) (Firth et al., 2021; McDonnell and Chapman, 2015). The results of CTmax experiments are also crucial as input variables for various modelling efforts used to inform management decisions, such as bioenergetics habitat modelling, species distribution modelling and cumulative threat risk assessments.
Historically, westslope cutthroat trout (Oncorhynchus clarkii lewisi) occurred in mountain lakes, headwater streams and into large rivers in southern Alberta, eastern Washington, Idaho, western Montana, eastern Oregon and northwestern Wyoming but currently the subspecies displays a fragmented distribution over large portions of its historic range (Behnke, 1992; Shepard et al., 2005). Several populations of westslope cutthroat trout are considered at risk under the Canadian Species at Risk Act and the US Endangered Species Act. The survival and recovery of threatened westslope cutthroat trout are jeopardized by the cumulative effects of increasing water temperature due to climate change, competition and hybridization from non-native trout species (i.e. rainbow trout), recreational fishing and habitat destruction due to development. Subsequently, this study is very timely for filling some of the knowledge gaps in the thermal limits of westslope cutthroat trout and may be important for provincial and federal entities managing the species by providing information on the physiology and habitat limitations of this species. The upper lethal temperature for westslope cutthroat trout has been described as lower (19.7°C) than for rainbow trout (24.4°C), but both species had similar optimum growth temperatures (13.6 and 13.1°C, respectively; Bear et al., 2007). A wide range of CTmax values have been reported in the literature depending on cutthroat trout subspecies, population, life stage and acclimation (De Staso and Rahel, 1994; Wagner et al., 2001; Underwood et al., 2012). Subsequently, we analyzed the metabolic rates and aerobic scope at two ecologically relevant temperatures (10°C and 15°C) of adult westslope cutthroat trout (Oncorhynchus clarkii lewisi), a species at risk in Canada, and adult rainbow trout (Oncorhynchus mykiss) an introduced congeneric species that competes with westslope cutthroat trout for resources. We also assessed upper critical temperature limits of adult westslope cutthroat trout via Tag, CTmax and the ‘CTmax–Tag window’, the difference between CTmax and Tag (Wells et al., 2016). A large CTmax-agitation window points toward an individual ceasing regular behavior and seeking refuge from the temperature increases earlier than an individual with a smaller window and provides an indication of the thermal buffer of the fish to escape thermally stressful conditions before losing equilibrium (Wells et al., 2016; Firth et al., 2021).
The specific objectives of this study were to (i) evaluate the effect of temperature on the metabolic rates (i.e. standard and maximum metabolic rates, aerobic scope) of westslope cutthroat trout and rainbow trout using intermittent-flow respirometry and (ii) analyze thermal thresholds for westslope cutthroat trout using behavioral CTmax experiments.
Materials and methods
We achieved our objectives by estimating the SMR, spontaneous maximum metabolic rate (MMRs) and forced maximum metabolic rate (MMRf) of westslope cutthroat trout and rainbow trout under two temperature acclimations and calculating the corresponding spontaneous aerobic scope (ASs) and forced aerobic scope (ASf). We also estimated CTmax and agitation temperature on westslope cutthroat trout acclimated to 10°C.
Experimental Animals
Westslope cutthroat trout eggs and milt were collected from the Fording River system in British Columbia, Canada (N 50°13′42.96″, W 114°51′39.71″) and fertilized at the aquatic fish holding facility of the Fisheries and Oceans Canada’s Freshwater Institute (Winnipeg, Manitoba, Canada) in July 2012. Fish used for respirometry experiments were first-generation (F1) gametes collected from the Fording River System. Fish used for CTmax experiments were the second generation (F2) bred from the Fording River system gametes. Juvenile rainbow trout were obtained from Lyndon Fish Hatcheries (New Dundee, Ontario, Canada) in April 2014. Upon arrival at Fisheries and Oceans Canada’s Freshwater Institute, fish weighed ~1 g.
All fish were held in 600-l flow-through tanks using de-chlorinated city water maintained at water temperatures of 10°C and 15°C and exposed to a 12:12 diurnal lighting regime with gradual light changes at 07:00 and 19:00 to mimic dawn and dusk. Fish were fed 0.5% of body weight using Hi-Pro Trout food #3 pellet (Hi-Pro Feeds, Okotoks, Alberta, Canada). Holding and experimental procedures were approved by the Freshwater Institute Animal Care Committee (Animal Use Protocols: FWI-ACC-AUP 2014-001, FWI-ACC-AUP 2015-001, FWI-ACC-AUP-2020-07) following the guidelines and recommendations outlined by the Canadian Council on Animal Care.
Intermittent-flow respirometry
System setup
An intermittent-flow respirometry system with four respirometry chambers (cylindrical plexiglass chambers; 14 cm in diameter × 45 cm in length, 7070 ml volume; Loligo® Systems Tjele, Denmark) was used to estimate SMR and MMRf of westslope cutthroat trout (AutoResp™ 2.2.0; Loligo®). The chambers were submerged in temperature-controlled tanks (300 l; 10.0°C ± 0.1°C or 15.0°C ± 0.1°C, depending on treatment) using water baths (Lauda Alpha, models RA 24 and A 24, Lauda-Königshofen, Germany). Oxygen concentrations in the tank were kept above 90% oxygen saturation using air stones.
The respirometry chambers were connected to two pumps (Universal 1048 Eheim, Deizisau, Germany) by non-toxic, Polyvinyl Chloride tubing. One pump recirculated water throughout the system and across an adjacent oxygen probe vessel. The other pump brought oxygenated water from the tank to the chamber and restored <90% oxygen saturation during the open flush phase. The pumps were controlled by AutoResp software (version 2.2; Loligo Systems, Tjele, Denmark) to produce a suite of opened and closed water circulation phases in the chambers. During the closed phase, only the recirculating pump was activated and no water or oxygen exchange with water outside of the respirometer chamber occurred. The oxygen depletion caused by fish and bacterial respiration was measured during this closed phase. The four oxygen probes (optical sensor dipping sensor, DP-PSt3-L2.5-ST10-YOP; precision ±0.05 mg O2∙l−1, PreSens, Regensburg Germany) were relayed to a multi-channel oxygen meter (OXY-4 mini, PreSens, Regensburg Germany) and run by the AutoResp software recording the oxygen concentrations.
Experimental procedure
Intermittent-flow respirometry experiments were conducted between January and March 2016. A total of 39 westslope cutthroat trout (mean ± S.D. body mass = 423.2 ± 104.7 g) and 32 rainbow trout (mean ± S.D. body mass = 438.9 ± 96.1 g) were used in the respirometry experiments. Fish were fasted for at least 24 h before experiments and were randomly selected from the treatment tank and transferred to a respirometry chamber. Fish were exposed to the same 12:12 diurnal cycle as during the fish-holding and isolated from any external stimuli with a dark curtain draped around all edges of the tank. Activity in the room where experiments were conducted was kept to a minimum.
Estimates of SMR, MMRf, MMRs, ASf and ASs were obtained for each fish. Estimation of SMR and MMRs was conducted first over a 48 h period (flush period, 5 min; wait period, 1 min; measuring period, 2–5 min). The measurement period varied depending on temperature and fish body mass to ensure that oxygen conditions remained normoxic in the chambers and to avoid oxygen concentrations below 7.6 mg O2·l−1, a critical value that may affect respiration rates (Tang et al., 2000). Consequently, a whole respirometry cycle lasted for 8–11 min. Following SMR, fish were exhausted using a standardized chase protocol of 5 min manual chase protocol consisting of a 1 min chase in a circular bucket, 3 min turning the fish over and 1 min holding the fish out of the water (similar to Roche et al., 2013). At the end of this 5 min chase, fish were not capable of burst swimming, a sign of exhaustion (Milligan, 1996). Immediately after chasing, fish were returned to the respirometry chamber and three oxygen consumption rate measurements were taken (ṀO2; see data analysis below). MMRf was defined as the single highest ṀO2 of the three estimates observed immediately after the chase procedure. MMRs was calculated as the highest single ṀO2 observed during the 24 h experiment (excluding MMRf measurements). Aerobic scopes between both SMR and MMRf (ASf) and SMR and MMRs (ASs) were calculated by subtracting the SMR from the MMRf and MMRs, respectively. Background bacterial oxygen demand (BOD) was estimated in each chamber by taking 10 min measurements of empty respirometry chambers immediately before and after each trial. The value of these BOD measurements was then subtracted from SMR and MMR estimates to adjust for BOD.
Critical thermal maximum CTmax
System setup
CTmax experiments were conducted from 30 November to 4 December 2020. For the CTmax experiment, two 200-l tanks were held at 10°C with aeration and two pumps (Universal 1048, Eheim, Deizisau, Germany) for circulation to ensure uniform heating. Each tank contained four 300 W titanium heating elements (TH-0300S titanium heaters, Finnex, Chicago, USA), which were used to heat the water during trials. Preliminary testing was conducted to select a water depth (24 cm) that ensured heaters consistently heated the water at a rate of 0.3°C·min−1. Westslope cutthroat trout were fasted for 24 h and placed in the same respirometry chambers as used in intermittent-flow respirometry experiments with the end caps removed and instead covered with soft mesh to allow for better water exchange with the tank water. Two fish per tank were left to acclimate overnight in the covered tank before starting a respective trial.
Experimental procedure
A total of 20 westslope cutthroat trout (mean ± S.D. body mass = 503.5 ± 140.9 g) were used for CTmax experiments. A given CTmax trial consisted of removing the tank cover, fish were then given 10 min to recover from being disturbed before the heaters were turned on and the trial began. Water temperatures were increased at a rate of 0.3°C min−1 and fish were constantly observed to monitor for abnormal behavior. The trial length was recorded, with temperature and dissolved oxygen levels being verified every 5 min to ensure that heating rates were constant and that dissolved oxygen saturations were >90%. Fish were monitored for two behavioural reactions, i.e. agitation temperature and CTmax. Agitation temperature was defined as the temperature at which fish displayed a sustained (>5 s) escape response behavior (McDonnell and Chapman, 2015). This escape response behavior was defined as ‘curling’ and ‘bursting’ movements being displayed. Curling was defined as the fish’s body being curved in a C shape as a result of attempting to turn around in the chamber and bursting behavior was defined as bursts of energetic swimming and was generally observed as sustained swimming into the netting enclosing the ends of the chamber. Both types of behavior were taken as an attempt to escape increasing water temperatures and subsequently the agitation temperature reflects physiological changes causing stress due to elevated water temperatures (McDonnell and Chapman, 2015).
CTmax was defined as a loss of equilibrium (LOE), which was defined as the endpoint of the trial (Beitinger et al., 2000; Morrison et al., 2020). Once LOE was reached, fish were removed from the treatment tank and quickly transferred to a recovery bath held at 10°C. Fish were left in the recovery bath overnight before being returned to a general population tank.
Data analysis
The oxygen consumption rate (ṀO2) was calculated as follows:
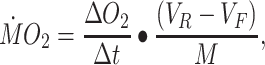 |
(1) |
where ṀO2 is the mass-specific oxygen consumption rate (mg O2·kg−1·h−1), 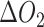 is the decline in oxygen content (mg/l) in the chamber,
is the decline in oxygen content (mg/l) in the chamber, 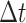 is the time elapsed during the closed measuring period (h), consequently
is the time elapsed during the closed measuring period (h), consequently 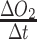 is the oxygen depletion slope, VR the water volume in the respirometer system (l), VF the fish volume (l) and M the fish body mass (kg).
is the oxygen depletion slope, VR the water volume in the respirometer system (l), VF the fish volume (l) and M the fish body mass (kg).
Visually inspecting ṀO2 by time graphs showed that westslope cutthroat trout had an habituation time of 10 ± 5 h. An example of typical patterns of ṀO2 of westslope cutthroat trout during respirometry experiments can be seen in Fig. 1.
Figure 1.
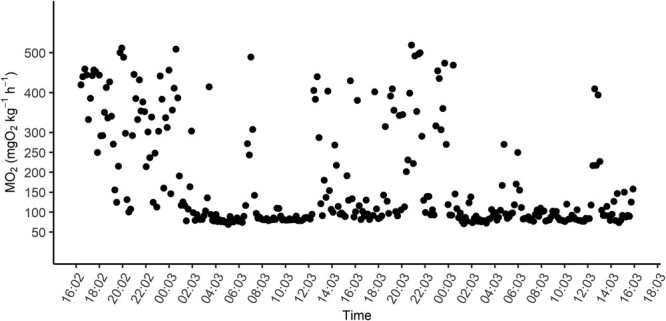
Oxygen consumption rate (ṀO2) of representative adult Westslope Cutthroat Trout over a 48 h respirometry experiment.
SMR was estimated as the q0·2 or mean of the lowest normal distribution of all ṀO2 estimates during the last 38 h of the experiment (Chabot et al., 2016). MMRs was calculated as the highest single ṀO2 observed during the 48 h experiment (excluding MMRf measurements; Svendsen et al., 2014). MMRf was defined as the single highest ṀO2 of the three estimates observed immediately after the chase procedure (Svendsen et al., 2012). Aerobic scopes between both, SMR and MMRs (ASs) and SMR and MMRf (ASf) were calculated by subtracting the SMR from MMRs and MMRf, respectively (Svendsen et al., 2012).
The mean temperatures where sustained agitation (>5 s), LOE and the difference between the two for all fish tested were used as the agitation temperature, CTmax and CTmax-agitation window (Beitinger et al., 2000).
Statistical analysis
Analyses of metabolic rates and CTmax experiments were conducted using R version 4.0.2 (R Foundation for Statistical Computing, 2020) and R Studio version 1.3.1056 (Rstudio PBC, 2020). Experimental data (SMR, MMRs, MMRf, ASs, ASf) was analyzed using two-way ANOVAs, with species and temperature as variables. Student’s t-tests were used to compare differences between MMRf and ASf, MMRs and ASs, as well as between-sex differences for CTmax. Residual plots were examined for normality and homogeneity among treatments and in all cases were found to meet assumptions. The full analysis of metabolic rate estimate comparisons can be found in Supplementary Table S1.
Results
Metabolic rates
Westslope cutthroat trout and rainbow trout had higher metabolic rates at 15°C compared to 10°C (Table 1; Fig. 2). SMR was different both between temperatures (P < 0.001) and species (P < 0.001). The intra-specific comparison found no difference in forced maximum metabolic rate (MMRf) between temperatures for westslope cutthroat trout (P = 0.059). However, MMRf was higher at 15°C compared to 10°C for rainbow trout (P < 0.001). Inter-specific comparisons showed lower MMRf for rainbow trout than westslope cutthroat trout at both 10°C and 15°C (P = 0.002 and P = 0.010, respectively).
Table 1.
Mean and standard deviation estimates of metabolic rates of adult Westslope cutthroat trout and rainbow trout at 10°C and 15°C
| Westslope cutthroat trout | Rainbow trout | |||
|---|---|---|---|---|
| Temperature (°C) | 10 | 15 | 10 | 15 |
| SMR (mg O2·kg−1·h−1) | 71.83 ±6.00 | 86.54 ±2.90 | 45.32 ±1.30 | 62.18 ±1.73 |
| MMRs (mg O2·kg−1·h−1) | 459.04 ± 11.90 | 560.62 ± 12.99 | 366.56 ± 16.25 | 444.85 ± 14.80 |
| MMRf (mg O2·kg−1·h−1) | 507.69 ± 24.59 | 564.95 ± 14.28 | 414.14 ±8.74 | 508.70 ± 14.85 |
| ASs (mg O2·kg−1·h−1) | 387.21 ± 11.22 | 474.26 ± 12.71 | 321.25 ± 15.30 | 382.68 ± 14.58 |
| ASf (mg O2·kg−1·h−1) | 435.86 ± 25.55 | 478.59 ± 14.86 | 368.83 ±7.86 | 441.88 ± 15.51 |
Figure 2.
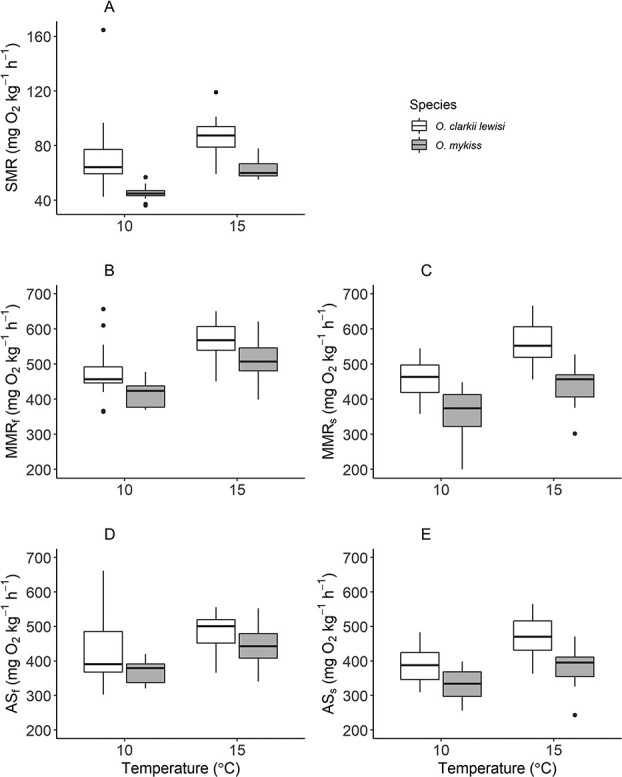
Metabolic rate estimates of adult Westslope Cutthroat Trout (Oncorhynchus clarkii lewisi) and Rainbow Trout (Oncorhynchus mykiss) tested at 10 and 15°C. (A) Standard metabolic rate (SMR), (B) Forced maximal metabolic rate (MMRf), (C) Spontaneous maximal metabolic rate (MMRs), (D) Forced aerobic scope (ASf), (E) Spontaneous aerobic scope (ASs).
Similarly, both species had higher MMRs at 15°C compared to 10°C (P < 0.001 and P = 0.002, respectively) and westslope cutthroat trout had higher MMRs than rainbow trout. MMRf was higher than MMRs for both species, with MMRf being different between test temperatures for rainbow trout (RNTR, 10°C: P = 0.003; 15°C: P = 0.004). However, these differences were not significantly different for westslope cutthroat trout (WSCT, 10°C: P = 0.087; 15°C: 0.764).
Forced aerobic scope (ASf) was not different between temperatures for westslope cutthroat trout (ANOVA; P = 0.170) but it was higher at 15°C for rainbow trout (P < 0.001). ASf was higher for westslope cutthroat trout at 10°C than rainbow trout (P = 0.026); however, no difference was observed at 15°C (P = 0.098). Spontaneous aerobic scope was found to be different at 10°C and 15°C for both species (WSCT: P < 0.001; RNTR: P = 0.007), as well as different between species at both test temperatures (10°C: P = 0.001; 15°C P < 0.001). Similar to MMR, ASf and ASs were not found to be different between 10°C and 15°C for westslope cutthroat trout (P = 0.087 and P = 0.764, respectively); however, ASf was higher for rainbow trout than ASs at both 10 and 15°C (P = 0.003 and P = 0.009, respectively).
Thermal limits
The critical thermal maximum of adult westslope cutthroat trout was 27.0 ± 0.8°C (mean ± S.D.), while the mean ± S.D. agitation temperature was 25.2 ± 1.0°C. The CTmax-agitation window for westslope cutthroat trout was relatively small with 1.8 ± 0.9°C. Only one fish did not recover from the CTmax experiments, with the mortality occurring shortly after being placed into the recovery bath. Of the 20 fish tested, we were able to identify the sex of 15 fish (9 males, 6 females, 5 unknown). Tag and CTmax were slightly higher for females than males, while the CTmax- Tag was slightly lower, with mean ± S.D. agitation temperature, CTmax, and CTmax- Tag window for females being 25.7 ± 0.8°C, 27.4 ± 0.6°C, and 1.7 ± 0.7°C, respectively, and 25.0 ± 0.9°C, 26.8 ± 1.0°C, and 2.0 ± 0.8°C, respectively, for males (Fig. 3). There was no difference between sexes for any of the comparisons (P = 0.200, P = 0.300, and P = 0.500, respectively).
Figure 3.
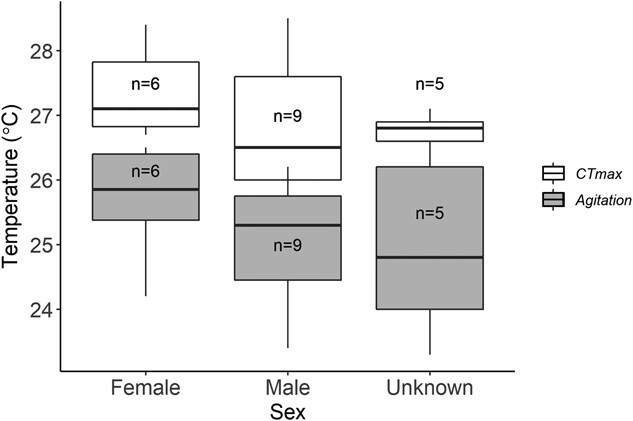
Critical Thermal Maximum (CTmax) and agitation temperature (Tag) estimates of adult Westslope Cutthroat Trout (Oncorhynchus clarkii lewisi) based on sex. Gray boxes display the Tag estimates, white boxplots display CTmax estimates. No difference was found for any metric between sexes (P = 0.200, P = 0.300, and P = 0.500, respectively)
Discussion
Due to climate change, westslope cutthroat trout may experience shifts in the distributions of their thermal habitat (Williams et al., 2009; Heinle et al., 2021). In order to simulate changes to species distributions and inform recovery strategies and actions, particularly for species at risk, it is important to understand a species’ physiology and thermal limits. The results from the intermittent-flow respirometry experiments on westslope cutthroat trout and its non-native competitor rainbow trout provide not only a parametrization of the temperature dependency of metabolic rates and aerobic scope but also valuable insights into inter-specific differences. Westslope cutthroat trout have a higher SMR and MMR than rainbow trout. Interestingly, at lower temperatures of 10°C westslope cutthroat trout had also a higher AS than rainbow trout, but no significant differences were observed at 15°C indicating a higher metabolic capacity of westslope cutthroat trout at lower temperatures (Fry, 1947; Pörtner and Farrell, 2008). Subsequently, the higher AS at lower temperature may indicate an advantage of westslope cutthroat trout over rainbow trout that could be compromised with increasing water temperatures due to climate change (Crozier et al., 2021).
A comparison of our mass-specific metabolic rate data to a previous intermittent-flow respirometry study on juvenile westslope cutthroat trout at the same temperatures showed a lower mean for SMR, MMRf and ASf in the adult fish in comparison to juveniles. A decrease in mass-specific metabolic rates is to be expected in larger, older individuals as growth and basic energetic demands decrease as organisms age (Hunt von Herbing and White, 2002; Killen et al., 2010). When comparing the rainbow trout metabolic rates to previous studies (Blake and Chan, 2006; Murray et al., 2017; Ekström et al., 2018), a similar decrease in metabolic rates as the fish increase in size was observed.
Albeit some evidence that chase protocols may underestimate MMR (Hvas and Oppedal, 2019; Raby et al. 2020), Little et al. (2020) demonstrated that for salmonids the here applied standardized chase protocol provided similar MMR estimates than those obtained with swim tunnels. Interestingly in our study, the MMR obtained using the standardized chase protocol resulted in similar rates to those estimated when analysing at the fish’s voluntarily displayed spontaneous MMR similar to the results by Zhang et al. (2020).
In regards to the critical thermal maximum experiments of westslope cutthroat trout acclimated to 10°C, CTmax of westslope cutthroat trout was slightly lower (i.e. 27.0 ± 0.8°C) than for the two co-occurring introduced, non-native salmonid species (i.e. rainbow trout and brook trout (Salvelinus fontinalis)) in the native range of westslope cutthroat trout. For example, estimates of CTmax for rainbow trout acclimated to ~10°C range from 27.6°C to 28.5°C (Lee and Rinne, 1980; Carline and Machung, 2001), while estimates for brook trout range from 28.2°C to 28.7°C for fish acclimated to ~10°C (Lee and Rinne, 1980; Carline and Machung, 2001). While both of these introduced species are considered to have a warmer water preference than westslope cutthroat trout are thought to, the similar CTmax values and small CTmax-agitation window exhibited by adult westslope cutthroat trout in our study suggest an ability to handle warmer water temperatures than previously assumed, as has also been suggested by thermal preference testing by Macnaughton et al. (2018, 2021). Westslope cutthroat started to become agitated at a temperature of 25.2 ± 1.0°C. While the high agitation temperature suggests an ability to withstand warmer water temperature for a short period before avoidance behaviour sets in, the small CTmax-agitation window (1.8 ± 0.9°C) may indicate detrimental sub-lethal thermal effects are initiating if the fish is unable to move to cooler thermal refugia quickly.
Further understanding of the thermal performance of westslope cutthroat trout will help conservation efforts of the species, both for understanding areas to protect as Critical Habitat, as well as to better understand how future climate change scenarios may affect the species distributions. More specifically, the results from this study will inform and parametrize cumulative effects assessments and minimal viable population analyses for the recovery planning of the species that are laid out in species’ recovery strategy and action plan and help delineate their critical habitat, respectively (DFO, 2019b).
Funding
This research was funded by Fisheries and Oceans Canada’s Species at Risk Program to ECE.
Data Availability
Data for this paper can be found at https://doi.org/10.5683/SP3/LFJ5NZ
Conflict of Interest
Not used.
Supplementary Material
Acknowledgments
We thank Laura Murray for assisting with the intermittent-flow respirometry experiments and Mélanie Aminot and Sarah Hnytka for helping with the CTmax experiments. We also thank Kerry Wautier for assistance in the fish rearing.
Contributor Information
Eva C Enders, Institute National de la Recherche Scientifique, Centre Eau Terre Environnement, Québec Québec, G1K 9A9, Canada.
Travis C Durhack, Fisheries and Oceans Canada, Freshwater Institute, Winnipeg Manitoba, R3T 2N6, Canada.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Bear EA, McMahon TE, Zale AV (2007) Comparative thermal requirements of westslope cutthroat trout and rainbow trout: implications for species interactions and development of thermal protection standards. Trans Am Fish Soc 136: 1113–1121. 10.1577/T06-072.1. [DOI] [Google Scholar]
- Becker CD, Genoway RG (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fish 4: 245–256. 10.1007/BF00005481. [DOI] [Google Scholar]
- Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fishes 58: 237–275. 10.1023/A:1007676325825. [DOI] [Google Scholar]
- Behnke RJ (1992) Native Trout of Western North America. merican Fisheries Society, Bethesda, MD, USA, pp. 275. [Google Scholar]
- Blake RW, Chan KHS (2006) Cyclic feeding and subsequent compensatory growth do not significantly impact standard metabolic rate or critical swimming speed in rainbow trout. J Fish Biol 69: 818–827. 10.1111/j.1095-8649.2006.01152.x. [DOI] [Google Scholar]
- Carline RF, Machung JF (2001) Critical thermal maxima of wild and domestic strains of trout. Trans Am Fish Soc 130: 1211–1216. . [DOI] [Google Scholar]
- Chabot D, Steffensen JF, Farrell AP (2016) The determination of standard metabolic rate in fishes. J Fish Biol 88: 81–121. 10.1111/jfb.12845. [DOI] [PubMed] [Google Scholar]
- Crozier LG, Burke BJ, Chasco BE, Widener DL, Zabel RW (2021) Climate change threatens Chinook salmon throughout their life cycle. Commun Biol 4: 222–214. 10.1038/s42003-021-01734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Staso J, Rahel FJ (1994) Influence of water temperature on interactions between juvenile Colorado River cutthroat trout and brook trout in a laboratory stream. Trans Am Fish Soc 123: 289–297. . [DOI] [Google Scholar]
- DFO (2019a) Review of Alberta Environment and Parks Cumulative Effects Assessment Joe Model. DFO Can Sci Adv Sec Sci Adv Rep 2019/045.
- DFO (2019b) Recovery Strategy and Action Plan for the Alberta Populations of Westslope Cutthroat Trout (Oncorhynchus larkia lewisi) in Canada 2019. [Proposed]. https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/recovery/westslope-cutthroat-trout-2019-proposed.html.
- Durhack TC (2020) Comparing metabolic rate estimates of two similar salmonids: Salvelinus confluentus and Salvelinus fontinalis. MSc Thesis, University of Manitoba. [Google Scholar]
- Durhack TC, Mochnacz NJ, Macnaughton CJ, Enders EC, Treberg JR (2021) Life through a wider scope: brook trout (Salvelinus fontinalis) exhibit similar aerobic scope across a broad temperature range. J Therm Biol 99: 102929. 10.1016/j.jtherbio.2021.102929. [DOI] [PubMed] [Google Scholar]
- Ekström A, Axelsson M, Gräns A, Brijs J, Sandblom E (2018) Importance of the coronary circulation for cardiac and metabolic performance in rainbow trout (Oncorhynchus mykiss). Biol Lett 14: 20180063. 10.1098/rsbl.2018.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason EJ, Farrell AP (2016) Oxygen uptake in Pacific salmon Oncorhynchus spp.: when ecology and physiology meet. J Fish Biol 88: 359–388. 10.1111/jfb.12790. [DOI] [PubMed] [Google Scholar]
- Firth LB, Harris D, Blaze JA, et al. (2021) Specific niche requirements underpin multidecadal range edge stability, but may introduce barriers for climate change adaptation. Divers Distrib 27: 668–683 [Google Scholar]
- Fry FEJ (1947) Effects of the environment on animal activity, 55. Publ Ont Fish Res Lab 1–62. [Google Scholar]
- Green EJ, Carrit DE (1967) New tables for oxygen saturation of sea water. J Mar Biol 25: 140–147. [Google Scholar]
- Heinle KB, Eby LA, Muhlfeld CC, Steed A, Jones L, D’Angelo V, Whiteley AR, Hebblewhite M (2021) Influence of water temperature and biotic interactions on the distribution of Westslope cutthroat trout (Oncorhynchus larkia lewisi) in a population stronghold under climate change. Can J Fish Aquat Sci 78: 444–456. 10.1139/cjfas-2020-0099. [DOI] [Google Scholar]
- Hunt von Herbing IH, White L (2002) The effects of body mass and feeding on metabolic rate in small juvenile Atlantic cod. J Fish Biol 61: 945–958. 10.1111/j.1095-8649.2002.tb01854.x. [DOI] [Google Scholar]
- Hvas M, Oppedal F (2019) Influence of experimental set-up and methodology for measurements of metabolic rates and critical swimming speed in Atlantic salmon Salmo salar. J Fish Biol 95: 893–902. 10.1111/jfb.14087. [DOI] [PubMed] [Google Scholar]
- Imholt C, Malcolm IA, Bacon PJ, Gibbins CN, Soulsby C, Miles M, Fryer RJ (2011) Does diurnal temperature variability affect growth in juvenile Atlantic Salmon Salmo salar? J Fish Biol 78: 436–448. 10.1111/j.1095-8649.2010.02838.x. [DOI] [PubMed] [Google Scholar]
- Killen SS, Atkinson D, Glazier DS (2010) The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol Lett 13: 184–193. 10.1111/j.1461-0248.2009.01415.x. [DOI] [PubMed] [Google Scholar]
- Lee AS, Koops MA (2020) Recovery potential modelling of Westslope cutthroat trout (Oncorhynchus clarkii lewisi) in Canada (Saskatchewan-Nelson River population). DFO Can Sci Adv Sec Sci Adv Rep 2020/046. iv+26. [Google Scholar]
- Lee RM, Rinne JN (1980) Critical thermal maxima of five trout species in the southwestern United States. Trans Am Fish Soc 109: 632–635. . [DOI] [Google Scholar]
- Little AG, Dressler T, Kraskura K, Hardison E, Hendriks B, Prystay T, Farrell AP, Cooke SJ, Patterson DA, Hinch SGet al. (2020) Maxed out: optimizing accuracy, precision, and power for field measures of maximum metabolic rate in fishes. Physiol Biochem Zool 93: 243–254. 10.1086/708673. [DOI] [PubMed] [Google Scholar]
- Macnaughton CJ, Durhack TC, Mochnacz NJ, Enders EC (2021) Metabolic performance and thermal preference of Westslope cutthroat trout Oncorhynchus clarkii lewisi and non-native trout across an ecologically relevant range of temperatures. Can J Fish Aquat Sci 78: 1247–1256. 10.1139/cjfas-2020-0173. [DOI] [Google Scholar]
- Macnaughton CJ, Kovachik C, Charles C, Enders EC (2018) Using the shuttlebox experimental design to determine temperature preference for juvenile Westslope cutthroat trout (Oncorhynchus clarkii lewisi). Cons Physiol 6: 1–10. 10.1093/conphys/coy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell LH, Chapman LJ (2015) At the edge of the thermal window: effects of elevated temperature on the resting metabolism, hypoxia tolerance, and upper critical thermal limit of a widespread African cichlid. Conserv Physiol 3: cov050. 10.1093/conphys/cov050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan CL (1996) Metabolic recovery from exhaustive exercise in rainbow trout. Comp Biochem Physiol A 113: 51–60. 10.1016/0300-9629(95)02060-8. [DOI] [Google Scholar]
- Morrison SM, Mackey TE, Durhack TC, Jeffrey JD, Lilian M, Mochnacz NJ, Hasler CT, Enders EC, Treberg JR, Jeffries KM (2020) Sub-lethal temperature thresholds indicate acclimation and physiological limits in brook trout Salvelinus fontinalis. J Fish Biol 97: 583–587. 10.1111/jfb.14411. [DOI] [PubMed] [Google Scholar]
- Munday PL, Donelson JM, Domingos JA (2017) Potential for adaptation to climate change in a coral reeffish. Glob Chang Biol 23: 307–317. 10.1111/gcb.13419. [DOI] [PubMed] [Google Scholar]
- Murray L, Rennie MD, Svendsen JC, Enders EC (2017) Respirometry increases cortisol levels in rainbow trout Oncorhynchus mykiss: implications for measurements of metabolic rate. J Fish Biol 90: 2206–2213. 10.1111/jfb.13292. [DOI] [PubMed] [Google Scholar]
- Naman SM, Rosenfeld JS, Neuswanger JR, Enders EC, Hayes JW, Goodwin EO, Jowett I, Eaton BC (2020) Bioenergetic habitat suitability curves for instream flow modelling: introducing user-friendly software and its potential applications. Fisheries 45: 605–613. 10.1002/fsh.10489. [DOI] [Google Scholar]
- Nelson JA (2016) Oxygen consumption rate v. rate of energy utilization of fishes: a comparison and brief history of the two measurements. J Fish Biol 88: 10–25. 10.1111/jfb.12824. [DOI] [PubMed] [Google Scholar]
- Pandit SN, Koirala L, Maitland BM, Poesch M, Enders EC (2017) Climate change risks, extinction debt, and conservation implications for an endangered freshwater fish carmine shiner (Notropis percobromus). Sci Total Environ 598: 1–11. 10.1016/j.scitotenv.2017.03.228. [DOI] [PubMed] [Google Scholar]
- Pörtner HO, Farrell AP (2008) Ecology. Physiology and climate change. Science 322: 690–692. 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- Raby GD, Doherty CLJ, Mokdad A, Pitcher TE, Fisk AT (2020) Post-exercise respirometry underestimates maximum metabolic rate in juvenilesalmon. Conserv Physiol 8: coaa063. 10.1093/conphys/coaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216: 2103–2110. 10.1242/jeb.082925. [DOI] [PubMed] [Google Scholar]
- RStudio Team (2020) RStudio: Integrated Development for R. RStudio PBC. Boston, MA. http://www.rstudio.com. [Google Scholar]
- Shepard BB, May BE, Urie W (2005) Status and conservation of westslope cutthroat trout within the Western United States. N Am J Fish Manag 25: 1426–1440 10.1577/M05-004.1. [DOI] [Google Scholar]
- Svendsen JC, Genz J, Anderson GW, Stol JA, Watkinson DA, Enders EC (2014) Evidence of circadian rhythm, oxygen regulation capacity, metabolic repeatability and positive correlations between forced and spontaneous maximal metabolic rates in lake sturgeon Acipenser fulvescens. PLoS One 9: e94693. 10.1371/journal.pone.0094693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen JC, Steffensen JF, Aarestrup K, Frisk M, Etzerodt A, Jyde M (2012) Excess posthypoxic oxygen consumption in rainbow trout (Oncorhynchus mykiss): recovery in normoxia and hypoxia. Can J Zool 90: 1–11. 10.1139/z11-095. [DOI] [Google Scholar]
- Tang M, Boisclair D, Ménard C, Downing J (2000) Influence of body weight, swimming characteristics, and water temperature on the cost of swimming in brook trout (Salvelinus fontinalis). Can J Fish Aquat Sci 57: 1482–1488. 10.1139/f00-080. [DOI] [Google Scholar]
- Underwood ZE, Myrick CA, Rogers KB (2012) Effect of acclimation temperature on the upper thermal tolerance of Colorado River cutthroat trout Oncorhynchus clarkii pleuriticus (Richardson). J Fish Biol 80: 2420–2433. 10.1111/j.1095-8649.2012.03287.x. [DOI] [PubMed] [Google Scholar]
- van der Lee AS, Koops MA (2020) Recovery potential modelling of westslope cutthroat trout (Oncorhynchus clarkii lewisi) in Canada (Saskatchewan-Nelson River population). DFO Can Sci Adv Sec Sci Adv Rep 2020/046: iv+26 p. https://waves-vagues.dfo-mpo.gc.ca/library-bibliotheque/40928810.pdf. [Google Scholar]
- Wagner EJ, Arndt RE, Brough M (2001) Comparative tolerance of four stocks of cutthroat trout to extremes in temperature, salinity, and hypoxia. West North Am Nat 61: 434–444. [Google Scholar]
- Wells ZRR, McDonnell LH, Chapman LJ, Fraser DJ (2016) Limited variability in upper thermal tolerance among pure and hybrid populations of a cold-water fish. Conserv Physiol 4: cow063. 10.1093/conphys/cow063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JE, Haak AL, Neville HM, Colyer WT (2009) Potential consequences of climate change to persistence of cutthroat trout populations. N Am J Fish Mgmt 29: 533–548. 10.1577/M08-072.1. [DOI] [Google Scholar]
- Zhang Y, Gilbert MJH, Farrell AP (2020) Measuring maximum oxygen uptake with an incremental swimming test and by chasing rainbow trout to exhaustion inside a respirometry chamber yields the same results. J Fish Biol 97: 28–38. 10.1111/jfb.14311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this paper can be found at https://doi.org/10.5683/SP3/LFJ5NZ


