Abstract
Aims
Although the number of patients suffering from heart failure with preserved ejection fraction (HFpEF) increases, the routine diagnosis remains a challenge. In the absence of a pathognomonic sign for HFpEF or specific treatment strategies, a prognosis‐based characterization of suspected patients remains promising for both the risk stratification of the patients and a disease definition. The Heart Failure Association (HFA) of the European Society of Cardiology has introduced an algorithm with different levels of likelihood regarding the diagnosis of HFpEF, the HFA‐PEFF score. We aimed to evaluate the predictive value of this algorithm in a large cohort regarding mortality, symptom burden, and the functional status.
Methods and results
DIAST‐CHF is a multicentre, population‐based, prospective, observational study in subjects with at least one risk factor for HFpEF between the age of 50 and 85. We calculated the HFA‐PEFF score (n = 1668) and analysed the risk groups for overall mortality, cardiovascular hospitalization, and submaximal functional capacity (6‐min walk distance) at baseline and after a follow‐up period of 10 years. Patients with high HFA‐PEFF score values 5&6 showed a higher mortality than those with an intermediate score (score values 2–4) and low score values (high 21.3% vs. intermediate 10.1% vs. low 4.3%, P < 0.001). Also, the burden of MACE (death, cardiovascular hospitalization, new myocardial infarction, first diagnosis of HF) was increased in the high score values group (high 40.7% vs. intermediate 25.9% vs. low 13.9%, P < 0.001). Similarly, patients with higher scores had higher cumulative incidences of cardiovascular hospitalizations (P = 0.011). Subjects with higher scores also had lower 6‐min walk distance both at baseline and during follow‐up.
Conclusions
The HFA‐PEFF score provides a reliable instrument to stratify suspected HFpEF patients by their risk for mortality, symptom burden, and functional status in cohort at risk with a follow‐up period of 10 years. As high HFA‐PEFF scores are associated with worse outcome, the HFA‐PEFF algorithm describes a defining approach towards HFpEF.
Keywords: Heart failure, Heart failure with preserved ejection fraction, HFpEF, Prognosis, HFA‐PEFF score
Introduction
Patients suffering from heart failure (HF) account for nearly 1–2% of the adult population in developed countries, rising up to ≥10% among people above 70 years of age with increasing prevalence due to demographic changes. 1 , 2 , 3 , 4 Patients suffering from HF with preserved ejection fraction (EF, HFpEF) account for nearly half of the HF population. 5 Despite remarkable progress in HF research, it remained controversial which criteria suffice to diagnose HFpEF additional to a preserved (>50%) left ventricular ejection fraction (LVEF). HF, like most degenerative diseases, is a ‘numerical disease’, defined by a change in relative function. 5 Whereas ‘categorical diseases’ are defined as the presence or absence of a pathogenic condition, for example, cancer or infections, numerical diseases are not defined by their presence but by the prognostic implications of an altered function. 5 The prognostic implication is either descriptive, a threshold of alteration associated with a worse prognosis, or therapeutic, when an intervention in prespecified setting is associated with an improved prognosis. HFpEF is a numerical disease as it results by the alteration of different characteristics, for example, diastolic function, left atrial (LA) dilatation, or LV hypertrophy. Because interventional strategies in major clinical trials have missed their hard clinical primary endpoint, the definition of HFpEF needs to be described by a multifactorial clinical approach to assess the prognosis. 6
Current guidelines updated the diagnosis of HFpEF formerly based on the assessment of echocardiographic surrogates for elevated intra‐cardiac filling pressures, serum biomarkers, and HF symptoms to the diagnostic approach of the HFA‐PEFF algorithm. 6
This new diagnostic algorithm, the HFA‐PEFF score, was proposed by the Heart Failure Association (HFA) of the European Society of Cardiology. 6 , 7 The HFA‐PEFF score is a multimodal approach based on a pre‐test assessment and a second step including the assessment by echocardiography and natriuretic peptides, functional testing, and aetiology diagnostics to estimate the likelihood (low, intermediate, or high) of suffering from HFpEF. A high likelihood score is considered diagnostic for HFpEF and a low‐likelihood score rules out HFpEF. The intermediate‐likelihood group requires exercise testing for further evaluation. The HFA‐PEFF score has been developed based on previous findings and expert opinion but was not based on calculations and prospectively performed cohorts to prove its clinical value and validity. So far, the HFA‐PEFF score has only been either retrospectively tested on small cohorts or with only a short follow‐up period. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15
An analysis with a sufficient follow‐up period to assess the prognostic value has been missing. Therefore, the aim of this study is to evaluate the diagnostic value of the HFA‐PEFF algorithm by assessing the predictive value of the score in a large well‐defined cohort with a long follow‐up period considering overall mortality symptom burden as well as functional status.
Methods
Study design and subject population
The observational Diagnostic Study on Prevalence and Clinical Course of Diastolic Dysfunction and Diastolic Heart Failure (DIAST‐CHF) is a multicentre population‐based prospective study within the framework of the German Competence Network for HF (CNHF) and the German Centre for Cardiovascular Research (DZHK). It is a unique database with detailed clinical and echocardiographic data to describe characteristics with a focus on HFpEF. The CNHF constitutes one of Europe's largest HF research programmes funded by the German Federal Ministry of Education and Research. Its rationale and design have been previously described. 16 , 17 The design of the CNHF and the many analyses of DIAST‐CHF have been previously published. 18 , 19 , 20 , 21 , 22 , 23 Briefly, the DIAST‐CHF investigated outpatients aged 50–85 years who were recruited between 2004 and 2006 with a history of overt HF or at least one risk factor. These risk factors included a history of HF, coronary disease, diabetes mellitus, sleep apnoea syndrome, or arterial hypertension. Candidates were referred by primary care physicians. The only exclusion criterion was unwillingness to participate, insufficient understanding of the German language, or unavailability for logistic reasons.
The study protocol was reviewed and approved by the institutional review board of each participating centre, and all patients provided written informed consent prior to enrolment. DIAST‐CHF was conducted in accordance with national laws, guidelines for good clinical practice, and the Declaration of Helsinki.
After study enrolment, all patients received a routine physical examination and a detailed cardiology assessment including extensive blood analyses and a transthoracic echocardiography. The follow‐up in‐person visits were planned after 12, 24, and 60 months as well as after 10 years. A telephone visit was performed after 9 years.
The HFA‐PEFF diagnostic algorithm
The HFA‐PEFF diagnostic algorithm is a clinical score including eight parameters in three categories (functional, morphological, and biomarker changes). These parameters include imaging signs for diastolic dysfunction, LV hypertrophy, left atrial dilatation, or increased natriuretic peptide levels. Each item is assigned to a category and is evaluated. Small changes will score 1 point, and larger changes 2 points. The possible range of the total score reaches from 0 to 6 points. Cumulative 5 or 6 points are considered diagnosed HFpEF. 0 or 1 point would rule out the presence of HFpEF. If the subject scored 2–4 points, stress testing is required to evaluate the presence of HFpEF.
Endpoint
The main aim of this analysis was to evaluate the prognostic value of the HFA‐PEFF algorithm in subjects with diagnosed HF or at least one risk factor for HFpEF with regard to assess the symptom burden, the functional status, and clinical outcome parameters.
For this purpose, we assessed (i) overall mortality; (ii) major adverse cardiac events (MACE) including death, cardiovascular hospitalization, new myocardial infarction, and first diagnosis of HF; and (iii) CV hospitalization.
Symptom burden and functional status were assessed by quality of life measures [measured by the 36‐Item Short Form Health Survey (SF‐36) questionnaire] and functional capacity measured by 6‐min walk test (6MWT).
We compared subjects with low vs. intermediate vs. high scores according to the proposed algorithm.
Statistical methods
Data preparation and descriptive statistics was performed by IBM SPSS, Version 28. We applied R, Version 4.1, inclusive the packages ggplot2, survival, and Hmisc to build multiple models and to generate graphs.
The study cohort was characterized by standard statistics: mean and standard deviation (SD) for continuous, count, and % for categorical characteristics. For three‐group comparisons of endpoints, we applied ANOVA with Dunnett's test as post hoc analysis. P ‐values were adjusted for multiple testing by the method of Bonferroni and Holm. 24
The 95% confidence intervals (CI) were calculated and displayed by longitudinal error bar plots. We calculated cumulative incidences of overall mortality by the Kaplan–Meier method, depicted it and tested it by log‐rank test. The association of common risk factors different between HFA‐PEFF groups in Table 1 was analysed by a multiple model. We started with the full linear regression model and simplified it by stepwise exclusion of variables using the Akaike Information Criterion (AIC). The effect estimates were determined with 95% CI. We calculated the concordance (c‐) statistics to assess the predictive power of our model.
Table. 1.
Study population baseline characteristics
| HFA‐PEFF score values 0–1 | HFA‐PEFF score values 2–4 | HFA‐PEFF score values 5 and 6 | |||||
|---|---|---|---|---|---|---|---|
| No HFpEF | Intermediate risk for HFpEF | HFpEF | P value | ||||
| n = 115 | n = 980 | n = 573 | Comparison across groups | ||||
| Mean/ | SD/ | Mean/ | SD/ | Mean/ | SD/ | ||
| Number | % | Number | % | Number | % | ||
| Age [years] | 57 | 7 | 65 | 8 | 70 | 8 | <0.001 |
| Female sex | 49 | 42.6% | 509 | 51.9% | 314 | 54.8% | 0.55 |
| BMI [kg/m2] | 27.2 | 4.8 | 28.7 | 4.8 | 29.1 | 4.8 | 0.002 |
| Waist–hip ratio | 0.93 | 0.09 | 0.94 | 0.21 | 0.94 | 0.13 | 1.0 |
| BP systolic [mmHg] | 135 | 18 | 145 | 20 | 151 | 23 | <0.001 |
| BP diastolic [mmHg] | 82 | 10 | 83 | 12 | 83 | 12 | 1.0 |
| HR [bpm] | 72 | 10 | 72 | 12 | 68 | 12 | 0.010 |
| Diabetes mellitus | 17 | 14.8% | 220 | 22.4% | 155 | 27.1% | 0.11 |
| Hypertension | 56 | 48.7% | 746 | 76.1% | 515 | 89.9% | <0.001 |
| Hyperlipidaemia | 19 | 16.5% | 384 | 39.2% | 258 | 45.0% | 0.000 |
| Hyperuricaemia | 9 | 7.8% | 112 | 11.4% | 97 | 16.9% | 0.027 |
| Non‐smoker a | 49 | 42.6% | 504 | 51.5% | 302 | 52.7% | 0.019 |
| Ex‐smoker | 40 | 34.8% | 356 | 36.4% | 220 | 38.4% | |
| Smoker | 26 | 22.6% | 118 | 12.1% | 51 | 8.9% | |
| Sleep apnoea | 4 | 3.5% | 55 | 5.6% | 35 | 6.1% | 1.0 |
| COPD | 8 | 7.0% | 74 | 7.6% | 39 | 6.8% | 1.0 |
| History of resuscitation | 0 | 0.0% | 20 | 2.0% | 9 | 1.6% | 1.0 |
| CAD | 10 | 8.7% | 127 | 13.0% | 145 | 25.3% | <0.001 |
| History of AMI | 5 | 4.3% | 64 | 6.5% | 59 | 10.3% | 0.12 |
| History of PCI | 6 | 5.2% | 73 | 7.5% | 79 | 13.9% | 0.001 |
| History of CABG | 0 | 0.0% | 26 | 2.7% | 41 | 7.2% | <0.001 |
| Atrial fibrillation | 4 | 3.5% | 57 | 5.8% | 44 | 7.7% | 1.0 |
| Diagnosis of heart failure | 4 | 3.5% | 87 | 8.9% | 83 | 14.5% | 0.002 |
| NYHA class a | 1.0 | ||||||
| I | 0 | 0.0% | 22 | 25.3% | 20 | 23.8% | |
| II | 4 | 100.0% | 47 | 54.0% | 44 | 52.4% | |
| III | 0 | 0.0% | 18 | 20.7% | 20 | 23.8% | |
| LV‐EF (%) | 63 | 6.0 | 61 | 6.4 | 61 | 6.5 | 0.036 |
| RV pacemaker | 0 | 0.0% | 9 | 0.9% | 13 | 2.3% | 0.39 |
| BV pacemaker | 0 | 0.0% | 1 | 0.1% | 0 | 0.0% | 1.0 |
| ICD | 0 | 0.00% | 1 | 0.10% | 1 | 0.17% | 0.86 |
| Years since diagnosis of HF | 0 | [0, 7.5] | 4 | [2, 10] | 3 | [0, 9] | |
| Median [quartiles] | |||||||
AMI, acute myocardial infarction; BMI, body mass index; BP, blood pressure; BV, biventricular; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; HR, heart rate; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; NYHA class: New York Heart Association functional class; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RV, right ventricular.
P‐values provided for not randomized groups; level of significance was set at 5%.
Delaney's and Vargha's A measurement of the effect size.
Cardiovascular hospitalization and death are competing risks. Thus, they were compared between the subgroups by means of cumulative incidences following Gray and Pepe.
Results
Subject population
A total of 1937 participants were included in DIAST‐CHF. For the present analysis, we excluded 269 subjects (n = 8 due to incomplete baseline characteristics and n = 174 due to missing or reduced LVEF values, n = 15 for valvular or congenital heart disease as well as n = 72 for missing natriuretic peptide levels), resulting in 1668 subjects for the complete analysis. Their baseline characteristics are presented in Table 1 .
In line with the proposed algorithm, we separated those with a low score (0–1 points) from those with an intermediate (2–4 points) or a high score (5–6 points). This resulted in 115 subjects in the low, 980 subjects in the intermediate, and 573 in the high score group. Subsequently, we compared all three groups.
Clinical outcomes and assessment of symptom burden and functional status
Overall mortality
The overall mortality at 10‐year follow‐up increases monotonically with the HFA‐PEFF score: low 5 (4.3%), intermediate 99 (10.1%), and high score 122 (21.3%), P < 0.001. Comparing the risk groups pairwise reconfirms this result: low vs. intermediate: P = 0.046; low vs. high: P < 0.001; intermediate vs. high: P < 0.001.
MACE
In line with the overall mortality, incidence of MACE grows monotonically across the groups: low 16 (13.9%), intermediate 254 (25.9%), and high score 233 (40.7%), P < 0.001. This effect is also shown in direct comparisons between the groups: low vs. intermediate score: P = 0.005; low vs. high score: P < 0.001; intermediate vs. high score: P < 0.001 (Figure 1 ).
Figure 1.
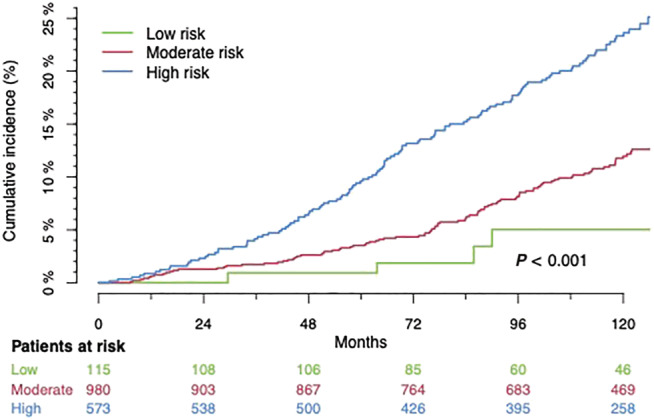
Cumulative overall mortality. Low score: score values 0 and 1; intermediate score: score values 2–4; high score: score values 5 and 6 in the HFA‐PEFF algorithm.
Cardiovascular hospitalization
A higher score was also associated with a higher number of cardiovascular hospitalizations per patient: low 0.10 [0.04, 0.17], intermediate 0.19 [0.15, 0.23], and high score 0.32 [0.26, 0.39], P < 0.001 in the global test. Whereas the difference in hospitalization rate between the low and the intermediate group was not significant (low vs. intermediate score: P = 0.25), the differences between the low‐risk and the high‐risk group as well as the intermediate‐risk and the high‐risk group were significant (low vs. high score: P = 0.003; intermediate vs. high score: 0.003).
The cumulative incidences of both MACE and cardiovascular hospitalization are shown in Figure S1 .
Functional capacity
Figure 2 shows the walking distance measured by the 6MWT both at baseline and at the 10‐year follow‐up (high vs. intermediate vs. low score at baseline: 588 ± 83 m vs. 558 ± 108 m vs. 537 ± 93 m, P < 0.001; low vs. intermediate score: P < 0.001; low vs. high score: P < 0.001; intermediate vs. high: P < 0.001). During 10‐year follow‐up, the differences between the groups grew: low 555 ± 76 m, intermediate 506 ± 116 m, and high score 473 ± 126 m (low vs. intermediate score: P = 0.006; low vs. high score: P < 0.001; intermediate vs. high score: P = 0.051).
Figure 2.

Six‐minute walking distance in all three groups at baseline and at 10‐year follow‐up. Low score: score values 0 and 1; intermediate score: score values 2–4; high score: score values 5 and 6 in the HFA‐PEFF algorithm. (A) Walk distance at baseline. (B) Walk distance at 10‐year follow‐up for the three groups. Results (average ± standard deviation): at baseline: low score 588 ± 83; median score 558 ± 108; high score 537 ± 93. At 10‐year follow‐up: low score 555 ± 76; median score 50 6 ± 116; high score 473 ± 126. Overall P value for changes over time: P < 0.001. Overall P value for differences between the groups at 10‐year follow‐up: P = 0.001. Low risk vs. median risk at 10‐year follow‐up: P = 0.006. Low risk vs. high risk: P = < 0.001. Median risk vs. high risk: P = 0.051.
Self‐rated physical function
Assessing physical limitations, we compared the physical functioning (PF) scale on the SF‐36 between the groups. Patients with high score values have lower PF values at baseline (low vs. intermediate vs. high score: 85.1 ± 18.9 vs. 76.5 ± 23.1 vs. 68.3 ± 25.8, overall comparison P < 0.001; low vs. intermediate score: P = 0.001; low vs. high score: P < 0.001; intermediate vs. high score: P < 0.001).
Self‐rated mental health
Comparing the mental health state by the mental health sub‐scale of the SF‐36 between the three groups showed a significant difference overall across the groups (low vs. intermediate vs. high score: 65.3 ± 18.1 vs. 62.3 ± 18.2 vs. 59.4 ± 18.0, overall comparison P = 0.004; low vs. intermediate score: P = 0.39; low vs. high score: P = 0.020; intermediate vs. high score: P = 0.018).
Analysis of the potential impact of single score items on prognosis in survival
As shown in Table 2 , the items included in the HFA‐PEFF score were analysed regarding their associations with mortality. Consistency of the score is confirmed, as major criteria are associated stronger than minor criteria with a higher risk for mortality. In our cohort, biomarkers and functional parameters showed stronger prognostic effects on the survival than morphological changes [HR 2.77 (1.99 3.85) for the major criterion of the biomarkers and HR 2.80 (1.55 5.07) for the major criterion of the functional parameters vs. HR 1.15 (0.66 2.01) for the major criterion of the morphological changes].
Table 2.
Association of single items of the HFA‐PEFF score on mortality
| Characteristic | Threshold | OR | 95% CI | |
|---|---|---|---|---|
| E/e′ 1 pt. | E/e′_mean 9–14.99 | 1.65 | 0.98 | 2.76 |
| E/e′ 2 pts. | E/e′_mean ≥ 15 | 2.03 | 1.01 | 4.09 |
| e′ 2 pts. |
Age <75 y: e′_sep < 7 cm/s, e′_lat < 10 cm/s; |
1.61 | 0.82 | 3.17 |
|
Age ≥75: e′_sep < 5 cm/s, e′_lat < 7 cm/s | ||||
| PASP > 35 | PASP > 35 mmHg | 2.17 | 1.03 | 4.56 |
| LAVi enlarged 1 pt. | LAVI > 29 mL/m2 | 1.50 | 0.85 | 2.62 |
| LAVi enlarged 2 pts. | LAVI > 34 mL/m2 | 3.20 | 1.85 | 5.54 |
| LVMi enlarged 1 pt. |
Women: ≥95 g/m2 or RWT > 0.42 Men: ≥115 g/m2 or RWT > 0.42 |
0.95 | 0.48 | 1.86 |
| LVMi enlarged 2 pts. |
Women: ≥122 g/m2 and RWT > 0.42 Men: ≥149 g/m2 and RWT > 0.42 |
1.03 | 0.53 | 1.98 |
| Biomarkers 1 pt. | Sinus rhythm: 125–220 pg/mL | 0.78 | 0.45 | 1.37 |
| Atrial fibrillation 375–660 pg/mL | ||||
| Biomarkers 2 pts. | Sinus rhythm: NT‐proBNP > 220 pg/mL | 1.44 | 0.88 | 2.36 |
| Atrial fibrillation: NT‐proBNP > 660 pg/mL | ||||
| RWT > 0.42 | RWT > 0.42 | 1.06 | 0.66 | 1.70 |
LAVI, left atrial volume index; PASP, pulmonary artery systolic pressure; RWT, relative wall thickness, calculated as twice the LV posterior wall thickness divided by the LV internal diameter at end‐diastole.
All items refer to the HFA‐PEFF score. 8
Multiple association of the risk factors with the HFA‐PEFF score
Figure 3 represents relevant clinical risk factors and their effect estimates on HFA‐PEFF score values. The illustrated parameters were the resulting relevant parameters after the reduction of baseline characteristics to a sparse model. The factors listed in Figure 3 including their effect estimates (and 95% CI) might provide an insight to the causes leading to the parameters assessed by the HFA‐PEFF score Table 3 .
Figure 3.
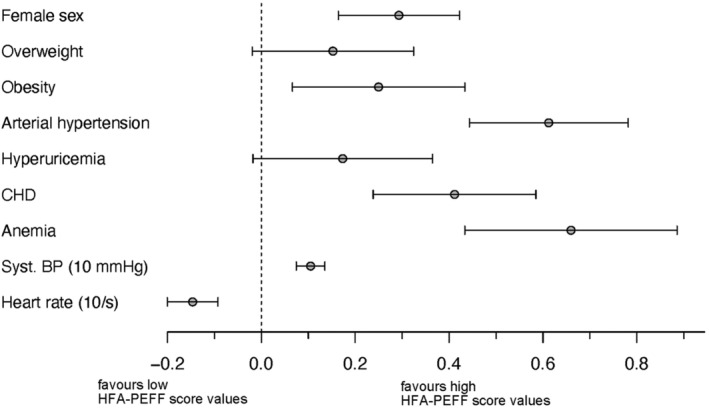
Risk factors associated with high score values. BP, blood pressure. Contribution of specific risk factors to the HFA‐PEFF score value if present. Female sex [0.29, 95% CI (0.16, 0.42)], that is, the presence of female sex is associated with a 0.29 points contribution to the HFA‐PEFF score value. Obesity [0.25 (0.07, 0.43)], arterial hypertension [0.61 (0.44, 0.78)], coronary heart disease [0.41 (0.24, 0.58)], anaemia [0.66 (0.43, 0.89)], systolic blood pressure [0.10 (0.07, 0.14) per 10 mmHg], and heart rate [per 10/min, −0.15 (−0.20, −0.09)].
Table 3.
Mortality, MACE, and CV hospitalization categorized by HFA‐PEFF score values during the 10‐year follow‐up
| HFA‐PEFF score value | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Dead | 0 | 5 | 8 | 40 | 51 | 70 | 52 | 226 |
| 0.0% | 5.2% | 7.4% | 9.5% | 11.4% | 19.4% | 24.5% | 13.5% | |
| MACE | 1 | 15 | 20 | 98 | 136 | 145 | 88 | 503 |
| 5.6% | 15.5% | 18.5% | 23.2% | 30.3% | 40.2% | 41.5% | 30.2% | |
| CV hospitalization | 1 | 10 | 8 | 51 | 67 | 71 | 37 | 245 |
| 5.6% | 10.3% | 7.4% | 12.1% | 14.9% | 19.7% | 17.5% | 14.7% | |
| Total | 18 | 97 | 108 | 423 | 449 | 361 | 212 | 1668 |
| 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | |
CV hospitalization, cardiovascular hospitalization; MACE, major adverse cardiac events including death, cardiovascular hospitalization, new myocardial infarction, and first diagnosis of HF.
Discussion
In a large prospective cohort of subjects with cardiovascular risk factors, we assessed the prognostic value of the HFA‐PEFF diagnostic algorithm regarding mortality and morbidity as well as relevant clinical measures. Higher scores were associated with a higher overall mortality, incidence of MACE, a higher symptom burden, and a lower the functional status. Considering the definition of numerical diseases, the HFA‐PEFF score identifies patients who have syndrome characterized by different altered parameters associated with a worse outcome. Therefore, the HFA‐PEFF score provides a reliable definition of HFpEF. The higher risk reflected by a higher score value may be explained by contributing known risk factors (Figure 3 ). Hence, the diagnostic HFA‐PEFF algorithm reflects high‐risk clinical compositions of altered parameters associated with a worse outcome.
This analysis gained power and robustness through a large number of subjects, prospectively investigated with an elaborated protocol to follow them up over 10 years.
Previous analyses of smaller cohorts assessed the HFA‐PEFF score with regard to hospitalization without mortality data as the primary endpoint over a follow‐up period of 5 years. 25 , 26 Selvaraj et al. as well as Aizpurua et al. confirmed that the high score groups assessed by the HFA‐PEFF algorithm suffered from a shorter hospitalization‐free survival. Our analysis confirmed higher hospitalization rates in subjects with high scores. They showed even a lower overall survival during a 10‐year follow‐up period.
Not only was the prognosis of patients with higher scores worse regarding mortality, MACE, and cardiovascular hospitalizations, but also the symptom burden and the functional status in these subjects were increased. Because functional capacity, for example, measured by the 6MWT, and worse prognosis are associated with higher score values, assessing the functional capacity is thought to be a parameter to identify high‐risk subjects within the HFpEF population. 27 The 6MWT is evaluated during the first step of the HFA‐PEFF algorithm, the pre‐test probability assessment, which triggers the next step, the assessment by the HFA‐PEFF score. The smaller decline in walking distance in the high score group is explained by the lower baseline values of this group. The two other groups were on a higher level at the beginning, implicating a higher chance of deterioration over time. Factors contributing to the restricted functional capacity include chronotropic incompetence, increased LV filling pressure, reduced cardiac output, and changes in the metabolism of peripheral muscular system. These reasons cannot be assessed in our population post hoc.
The DIAST‐CHF cohort is close to a real‐world scenario because its inclusion criteria are very broad and there are no cardiovascular exclusion criteria.
58.8% of the subjects from the DIAST‐CHF study qualified for the intermediate score group. Therefore, most subjects of our analysed cohort would require a diastolic stress testing strategy with regard to the HFA‐PEFF algorithm. This requirement of further testing challenges the current common practice as well as current care provider frameworks. Most probably, our cohort overestimates the number of subjects with intermediate scores because the study design augmented an at‐risk population without signs or symptoms of HF. Future cohorts need to reassess this distribution by including parameters from the HFA‐PEFF score at baseline.
Clinical implications
All studies evaluating the proposed diagnostic algorithm are challenged by the fact that there is no gold standard but expert opinion in diagnosing HFpEF. The HFA‐PEFF algorithm provides an HFpEF definition characterized by its prognostic value. The disease definition allows further investigation of the HFpEF population with multiple advantages: high event rates for interventional trials as well as higher comparability of trial and study data by better characterization.
However, complex algorithms like the HFA‐PEFF algorithm have a high threshold to be implemented in clinical or trial routine.
Limitations
The DIAST‐CHF study was designed before the introduction of the HFA‐PEFF score. Thus, not all components of the score were fully documented.
Within the intermediate score group, the HFA‐PEFF algorithm suspected subjects both suffering and not suffering from HFpEF, distinguished by a diastolic stress examination. These stress examinations were not performed within DIAST‐CHF. Therefore, we could not further analyse the differences within the intermediate score group.
SF‐36 values were captured only in a relatively small number of patients. Moreover, the study population is at risk to develop HF, whereas the algorithm is primarily designed to assess the presence of HFpEF. Because the work‐up for the intermediate HFA‐PEFF group is not established routinely yet, invasive testing is often required but not routinely implemented.
Funding
This work was supported by the German Competence Network for Heart Failure, funded by the German Federal Ministry of Education and Research, the Charité – Universitätsmedizin Berlin, Germany, and the German Centre for Cardiovascular Research (Deutsches Zentrum für Herz‐Kreislaufforschung e.V., DZHK ‐ funding number: 81X3100214).
Conflict of interest
No conflict of interest was declared regarding the presented analysis. BP, FE and CT are authors of the HFA‐PEFF consensus recommendation statement by the HFA/ESC. Honoraria and consultancy fees for pharmaceutical companies did not interfere with this analysis for any author.
Supporting information
Figure S1. Cumulative incidences for death and cardiovascular hospitalization. Low score: score values 0 and 1; intermediate score: score values 2–4; high score: score values 5 and 6 in the HFA‐PEFF algorithm. Times were truncated at 126 months.
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Hashemi, D. , Mende, M. , Trippel, T. D. , Petutschnigg, J. , Hasenfuss, G. , Nolte, K. , Herrmann‐Lingen, C. , Feuerstein, A. , Langhammer, R. , Tschöpe, C. , Pieske, B. , Wachter, R. , and Edelmann, F. (2022) Evaluation of the HFA‐PEFF Score: results from the prospective DIAST‐CHF cohort. ESC Heart Failure, 9: 4120–4128. 10.1002/ehf2.14131.
References
- 1. Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004; 351: 2049–2057. [DOI] [PubMed] [Google Scholar]
- 2. Bleumink GS, Knetsch AM, Sturkenboom MCJM, Straus SMJM, Hofman A, Deckers JW, Witteman JCM, Stricker BHC. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure ‐ The Rotterdam study. Eur Heart J. 2004; 25: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 3. Redfield MM, Jacobsen SJ, Burnett JJC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA. 2003; 289: 194–202, 202. [DOI] [PubMed] [Google Scholar]
- 4. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart Coats AJ. Validating the HFA‐PEFF score ‐ or how to define a disease? Eur J Heart Fail. 2020; 22: 428–431. [DOI] [PubMed] [Google Scholar]
- 6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JG. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 7. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSPP, Lancellotti P. How to diagnose heart failure with preserved ejection fraction: The HFA‐PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 8. Barandiarán Aizpurua A, Sanders‐van Wijk S, Brunner‐La Rocca H‐P, Henkens M, Heymans S, Beussink‐Nelson L, Shah SJ, van Empel VPM. Validation of the HFA‐PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020; 22: 413–421. [DOI] [PubMed] [Google Scholar]
- 9. Parcha V, Malla G, Kalra R, Patel N, Sanders‐van Wijk S, Pandey A, Shah SJ, Arora G, Arora P. Diagnostic and prognostic implications of heart failure with preserved ejection fraction scoring systems. ESC Heart Fail. 2021; 8: 2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sotomi Y, Iwakura K, Hikoso S, Inoue K, Onishi T, Okada M, Fujii K, Okamura A, Tamaki S, Yano M, Hayashi T, Nakagawa A, Nakagawa Y, Nakatani D, Yasumura Y, Yamada T, Sakata Y, the OCVC‐Heart Failure Investigators . Prognostic significance of the HFA‐PEFF score in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2021; 8: 2154–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tada A, Nagai T, Omote K, Iwano H, Tsujinaga S, Kamiya K, Konishi T, Sato T, Komoriyama H, Kobayashi Y, Takenaka S, Mizuguchi Y, Sato T, Yamamoto K, Yoshikawa T, Saito Y, Anzai T. Performance of the H(2)FPEF and the HFA‐PEFF scores for the diagnosis of heart failure with preserved ejection fraction in Japanese patients: A report from the Japanese multicenter registry. Int J Cardiol. 2021; 342: 43–48. [DOI] [PubMed] [Google Scholar]
- 12. Nikorowitsch J, Bei der Kellen R, Kirchhof P, Magnussen C, Jagodzinski A, Schnabel RB, Blankenberg S, Wenzel J‐P. Applying the ESC 2016, H(2) FPEF, and HFA‐PEFF diagnostic algorithms for heart failure with preserved ejection fraction to the general population. ESC Heart Fail. 2021; 8: 3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun Y, Si J, Li J, Dai M, King E, Zhang X, Zhang Y, Xia Y, Tse G, Liu Y. Predictive value of HFA‐PEFF score in patients with heart failure with preserved ejection fraction. Front Cardiovasc Med. 2021; 8: 656536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egashira K, Sueta D, Komorita T, Yamamoto E, Usuku H, Tokitsu T, Fujisue K, Nishihara T, Oike F, Takae M, Hanatani S, Takashio S, Ito M, Yamanaga K, Araki S, Soejima H, Kaikita K, Matsushita K, Tsujita K. HFA‐PEFF scores: Prognostic value in heart failure with preserved left ventricular ejection fraction. Korean J Intern Med. 2022; 37: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amanai S, Harada T, Kagami K, Yoshida K, Kato T, Wada N, Obokata M. The H(2)FPEF and HFA‐PEFF algorithms for predicting exercise intolerance and abnormal hemodynamics in heart failure with preserved ejection fraction. Sci Rep. 2022; 12: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehrhof F, Löffler M, Gelbrich G, Özcelik C, Posch M, Hense HW, Keil U, Scheffold T, Schunkert H, Angermann C, Ertl G. A network against failing hearts‐Introducing the German “Competence Network Heart Failure”. Int J Cardiol. 2010; 145: 135–138. [DOI] [PubMed] [Google Scholar]
- 17. Hashemi D, Blum M, Mende M, Störk S, Angermann CE, Pankuweit S, Tahirovic E, Wachter R, Pieske B, Edelmann F, Düngen HD, the German Competence Network for Heart Failure . Syncopes and clinical outcome in heart failure: Results from prospective clinical study data in Germany. ESC Heart Fail. 2020; 7: 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer T, Chavanon M‐L, Herrrmann‐Lingen C, Roggenthien M, Nolte K, Pieske B, Wachter R, Edelmann F. Elevated plasma C‐terminal endothelin‐1 precursor fragment concentrations are associated with less anxiety in patients with cardiovascular risk factors. Results from the observational DIAST‐CHF study. PLoS ONE. 2015; 10: e0136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edelmann F, Stahrenberg R, Polzin F, Kockskämper A, Düngen H‐D, Duvinage A, Binder L, Kunde J, Scherer M, Gelbrich G, Hasenfuß G, Pieske B, Wachter R, Herrmann‐Lingen C. Impaired physical quality of life in patients with diastolic dysfunction associates more strongly with neurohumoral activation than with echocardiographic parameters: Quality of life in diastolic dysfunction. Am Heart J. 2011; 161: 797–804. [DOI] [PubMed] [Google Scholar]
- 20. Bobenko A, Duvinage A, Mende M, Holzendorf V, Nolte K, Herrmann‐Lingen C, Binder L, Düngen H‐D, Hasenfuss G, Pieske B, Wachter R, Edelmann F. Outcome assessment using estimation of left ventricular filling pressure in asymptomatic patients at risk for heart failure with preserved ejection fraction. IJC Heart Vasc. 2020; 28: 100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haarmann H, Koch J, Bonsch N, Mende M, Werhahn SM, Lüers C, Stahrenberg R, Edelmann F, Holzendorf V, von Haehling S, Pieske B, Andreas S, Lüthje L, Wachter R. Morbidity and mortality in patients with cardiovascular risk factors and obstructive sleep apnoea: Results from the DIAST‐CHF cohort. Respir Med. 2019; 154: 127–132. [DOI] [PubMed] [Google Scholar]
- 22. Stahrenberg R, Duvinage A, Mende M, Gelbrich G, Auf der Heide W, Düngen H‐D, Binder L, Nolte K, Herrmann‐Lingen C, Hasenfuß G, Pieske B. Determinants of submaximal exercise capacity in patients at risk for heart failure with preserved ejection fraction‐results from the DIAST‐CHF study. ESC Heart Fail. 2015; 2: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lüers C, Trippel TD, Seeländer S, Wachter R, Hasenfuss G, Lindhorst R, Bobenko A, Nolte K, Pieske B, Edelmann F. Arterial stiffness and elevated left ventricular filling pressure in patients at risk for the development or a previous diagnosis of HF‐A subgroup analysis from the DIAST‐CHF study. J Am Soc Hypertens. 2017; 11: 303–313. [DOI] [PubMed] [Google Scholar]
- 24. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979; 6: 65–70. [Google Scholar]
- 25. Barandiarán Aizpurua A, Sanders‐van Wijk S, Brunner‐La Rocca H‐P, Henkens M, Heymans S, Beussink‐Nelson L, Shah SJ, van Empel VPM. Validation of the HFA‐PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. 2019: 1–9. [DOI] [PubMed] [Google Scholar]
- 26. Selvaraj S, Myhre PL, Vaduganathan M, Claggett BL, Matsushita K, Kitzman DW, Borlaug BA, Shah AM, Solomon SD. Application of diagnostic algorithms for heart failure with preserved ejection fraction to the community. JACC Heart Fail. 2020; 8: 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nayor M, Houstis NE, Namasivayam M, Rouvina J, Hardin C, Shah RV, Ho JE, Malhotra R, Lewis GD. Impaired exercise tolerance in heart failure with preserved ejection fraction: Quantification of multiorgan system reserve capacity. JACC Heart Fail. 2020; 8: 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cumulative incidences for death and cardiovascular hospitalization. Low score: score values 0 and 1; intermediate score: score values 2–4; high score: score values 5 and 6 in the HFA‐PEFF algorithm. Times were truncated at 126 months.


