Abstract

This paper presents an investigation of the feasibility of intercalating lignocellulose/xanthan gum (XG) and organic polymers into bentonite to obtain an efficient fire extinguishing gel material. The bentonite-based hybrid gel was prepared by adding polyacrylates, Al(OH)3, lignocellulose, and XG into a bentonite suspension, and the resulting gel was characterized. The results showed that no cracking and powdering were found on the surface of the hybrid gel due to the formation of the cross-linked network in the bentonite, and a wide mesopore size distribution and good thermal stability were observed. The hybrid gel also exhibits a wide range of water adsorption ratios, excellent water retention, adjustable gelation times, shear thinning characteristics, and improved compressive strength (the yield stress reaches up to 13 MPa). Based on these characterizations, the mechanism of hybrid gel formation is proposed. The inhibition performance of the hybrid gel on coal spontaneous combustion indicates that the addition of the gel slows down the oxygen chemisorption and thus increases the ignition temperature. Due to the presence of the hybrid gel in the coal, the crossing point temperatures were increased and the lowest CO concentration was produced.
Introduction
Coal is the most abundant and widely distributed basic energy source in the world, playing an important role in global economic and social development.1−3 However, coal is subject to spontaneous combustion in all aspects of mining, transportation, and storage, posing a serious threat to the health of personnel, damage to equipment and instruments, and waste of coal resources.4−8 Therefore, it is imperative to prevent and control coal spontaneous combustion.
It is well known that coal spontaneous combustion is the result of oxidation taking place between coal and oxygen, which releases a large amount of heat and thus raises the temperature of the coal.9−11 Therefore, the key to prevent and control coal spontaneous combustion is to isolate the contact coal from air, reduce air leakage, and lower the temperature of the coal body. Due to its large heat capacity and adhesion, gel fire extinguishing material has advantages in lowering coal temperature, isolating coal from air and sealing air leakage, and so forth. It has achieved great success in the field of underground coal seam fire extinguishing and preventing spontaneous combustion of floating coal and become an effective means to prevent and extinguish coal seam fires.12−15 The existing gel fire-extinguishing materials are mainly classified into inorganic and organic gels. Silicate gel formed by liquid water glass and ammonium salt or sodium bicarbonate is the most widely used inorganic gel in Chinese coal mines.16−19 Although silicate gel has a certain fire extinguishing effect, some disadvantages, such as producing harmful gas, poor compressive strength, uncontrolled gelation time, easy powder and dry cracking after water loss, and so forth, also limit its application. Unlike inorganic gels, organic gels are formed by the polymerization of low molecular mass hydrophilic organic compounds, usually semi-solid, where the organic liquid phase is immobilized within a mesh/network of gelling agent aggregates.20−24 The organic gels exhibit viscoelasticity and thermal stability. Meanwhile, a large amount of water can be stored in the cross-linked three-dimensional network of the organo-hydrogel, which can make the organo-hydrogel have a strong water absorption and retention capacity. The efficient water retention and thermostability of organic hydrogels have attracted many researchers to study the synthesis and properties to prevent and control coal oxidation.25−28 However, organic gels also have some disadvantages, such as high cost, harsh experimental conditions, and poor flowability, which limit their application in coal mine fire protection. Therefore, the synthesis of fire extinguishing gel materials with low cost and excellent fire extinguishing properties remains a challenge.
Bentonite consists mainly of montmorillonite and has a layered silica structure. Early reports indicate that bentonite can be mixed or intercalated with polymer systems to form good matrices for preparing hybrid gels.29−31 Recently, polymer hybrids by mixing bentonite and organic polymer for applications in many different fields have been fully developed.32−34 Plenty of polymer–bentonite nanocomposite hydrogels with improved mechanical and adsorption properties, such as chitosan–poly(vinyl alcohol)/bentonite nanocomposites,35 polyacrylamide/montmorillonite hydrogel,36,37 and polymethyl(methacrylate)–bentonite hybrid,38 have been prepared. Moreover, even water glass/coagulant/polymer plasticizer/bentonite plastogel was prepared and used to prevent and control fires in coal mines,39 disadvantages such as uncontrolled gelation times and the high cost of organic gels still exist. These have severely limited the practical application of bentonite-containing gel fireproofing materials in coal mines.
Xanthan gum (XG) is an anionic polysaccharide composed of glucose, mannose, and glucuronic acid. XG solutions using trivalent metal ions as crosslinking agents can produce hydrogels and have been used in the drilling industry and the food industry.40−42 Meanwhile, lignocellulose is the main component of plant biomass, which is an abundant, renewable, and sustainable biological resource on earth. The main components of lignocellulose are cellulose, lignin, and hemicellulose. As a result, lignocellulose has been widely used in the wood products industry and the pulp and paper industry.43−45 Particularly, lignocellulose-derived hydrogels such as lignin-containing cellulose nanofibril-reinforced polyvinyl alcohol hydrogels, PPy/cellulose hybrid hydrogel, and PAM/polyanionic cellulose composite hydrogel have been synthesized and applied mainly in flexible solid-state supercapacitors.46−48 The non-toxic, biodegradable, biocompatible XG and lignocellulose make them very compatible with green chemistry.
Disparate studies in the literature suggest that bentonite, XG, and lignocellulose can enhance some properties of hydrogels. However, what remains to be demonstrated is whether intercalation of XG/lignocellulose and organic polymer systems in bentonite can result in a hybrid gel fire extinguishing material with controlled gelation time and excellent refractory properties. In this work, we investigate the feasibility of intercalating XG/lignocellulose and organic polymer into bentonite to obtain an efficient fire extinguishing gel material with controlled gelation time. The effects of XG, lignocellulose, and organic polymer addition on the surface structure and properties of the hybrid gel were investigated, and the inhibition of coal spontaneous combustion by the hybrid gel was studied.
Experimental Section
Raw Materials and Reagents
Sodium bentonite was purchased from Jiangsu Mufeng Bentonite Mining Co., Ltd. The composition of the bentonite sample is shown in Table 1. Lignocellulose was purchased from Shijiazhuang Xinyuan Cellulose Co., Ltd. Food-grade XG was purchased from Hebei Yan Xing Chemical Co., Ltd. Sodium polyacrylate (PAAs, industrial grade), aluminum hydroxide [Al(OH)3, A.R. grade], calcium oxide (CaO, A.R. grade), and sodium silicate (Na2SiO3, industrial grade) were purchased from local medical stations.
Table 1. Chemical Composition of Bentonite Sample in wt %.
| composition | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | Fe2O3 |
|---|---|---|---|---|---|---|---|
| wt/% | 3.89 | 1.86 | 14.30 | 54.60 | 0.69 | 2.70 | 5.46 |
The coal samples were collected from Shenshupan (SSP) Colliery in Shaanxi Province, Xutuan (XT) Colliery in Anhui Province, and Huoerxinhe (HEXH) Colliery in Shanxi Province. The proximate analysis for the coal samples was carried out according to the American Society for Testing Material (ASTM), with approximately 1 g of coal sample used to estimate the moisture, ash, and volatile matter contents. The fixed carbon is calculated by subtracting (ash content + volatile matter + moisture content) from 100%, and the results are summarized in Table 2.
Table 2. Proximate Analysis of Coal Samples.
| proximate
analysis (air dried basis) (%) |
||||
|---|---|---|---|---|
| sample | Mad | Aad | Vad | FCad |
| SSP coal | 6.94 | 0.92 | 36.21 | 55.93 |
| XT coal | 1.54 | 16.81 | 31.26 | 50.39 |
| HEXH coal | 0.88 | 27.40 | 8.96 | 63.32 |
Preparation of Bentonite-Based Hybrid Gel
First, a bentonite suspension was prepared by dispersing the bentonite into the water, and then, the dispersion was stirred well to ensure that the bentonite particles were uniformly dispersed in the solution. Subsequently, different weights of PAAs and gelling agents were added to the bentonite suspension, followed by magnetic stirring at room temperature. Thereafter, XG and lignocellulose solutions of different concentrations were prepared by dissolving XG and lignocellulose in water under stirring and then manually mixed with bentonite/PAAs mixture. The final mixture was processed under vigorous stirring to form a bentonite-based hybrid gel.
In order to obtain inorganic gels of bentonite, it is generally necessary to add a suitable gelling agent to the bentonite suspension. At present, the commonly used gelatinizing agent of bentonite inorganic gels mainly includes some inorganic salts containing high metal ions (MgO, CaO, and Al2O3) and cellulose polymers (sodium carboxymethylcellulose).49,50 However, the PAA polymer used in our experiment has poor salt tolerance, and divalent ions such as Ca2+ and Mg2+ would reduce the PAAs polymer’s water sorption properties and make it precipitate out of the colloidal suspension. Therefore, neither MgO nor CaO can be used as a gelatinizing agent in this study.
It is found that although the CaO/Na2SiO3 system contains Ca2+ and Na+, CaO can be hydrolyzed to Ca(OH)2, which can react with Na2SiO3 to generate calcium silicate hydrate (CSH).51 CSH gel can bind bentonite particles together and promote the formation of bentonite gel, so CaO/Na2SiO3 was first selected as the gelatinizing agent for the preparation of hybrid gel. In addition, aluminum hydroxide [Al(OH)3] is widely used in medicine, which can be dispersed in water to form a suspended gel.52 Therefore, Al(OH)3 is also selected as a possible gelling agent in this study.
Two types of bentonite-based hybrid gels were synthesized by mixing bentonite (8 g), PAAs (0.3 g), and XG (0.1 g) and lignocellulose (2 g), CaO (0.5 g)/Na2SiO3 (0.35 g), and Al(OH)3 (0.5 g) were added to the reaction system as gelatinizing agents, respectively, and the effects of CaO/Na2SiO3 and Al(OH)3 on the stability of the hybrid gel were then investigated. To our surprise, although the two hybrid gels were obtained successfully, a lot of water seeped out of the hybrid gel that used CaO/Na2SiO3 as a gelling agent after it was left in room temperature for 3 days. This may be due to excess Ca2+ in CaO/Na2SiO3 systems still affecting the PAAs polymer’s water sorption property, so CaO/Na2SiO3 is not suitable for the reaction system. On the contrary, the other hybrid gel that used Al(OH)3 as a gelatinizing agent is very stable, and no water seepage phenomena occurred. Therefore, Al(OH)3 was chosen as the optimal gelling agent for the synthesis of hybrid gel in this study.
Testing and Characterization Methods
Test of Gelation Time
The gelation time was measured by the bottle test, that is, the components were mixed in the sequence. After the mixture was fully stirred using an electric mixer, the time measurement was immediately started and the flow state of the slurry in the beaker was observed. When the position of the gel did not move upon inversion of the beaker containing the gel slurry, the timing was stopped and the time shown by the stopwatch was recorded, which is the gelation time. Each group was tested three times and the average measurement was obtained.
Water Sorption Behaviors of Dried Hybrid Gel
The water sorption behavior of the composite gel was tested using the weighing method, concerning our previous work,26 as follows. The prepared hybrid gels were lyophilized and then milled into particles of approximately 1 mm in size. Each dried sample was milled into powders of 150–178 μm before use. The samples whose weights were recorded as W0 were then placed in a beaker filled with enough water for 12 h to ensure that the hybrid gels fully absorb the water. Afterward, remove excess moisture from the surface of each sample. Weights were now recorded as W1. The water-absorbing rate (WAR) of the hybrid gel can be described using the following equation (eq 1)
| 1 |
Subsequently, the saturated gel samples were placed in a ventilated environment at room temperature. The samples were weighed every day and the weights were recorded as Wt. The water retention rate (WR) of the gel was calculated using the following equation (eq 2)
| 2 |
Microstructure Characterization
The surfaces of the dried samples were sprayed with gold and the morphology of the lyophilized samples was observed under a Quanta 250 scanning electron microscope (SEM) (FEI Company, USA). In addition to the surface morphology, the elemental distribution on the surface was detected and mapped using an energy-dispersive X-ray spectrometer (EDS, X-Max). The mineralogical analysis of the raw materials and the prepared hybrid gel was examined by X-ray diffraction (XRD) using a Bruker Discovery D-8 X-ray diffractometer with Cu Kα radiation in the 2θ range of 3–70°. Moreover, sodium bentonite and bentonite-based hybrid gel prepared were also characterized by FTIR spectra on Nicolet 6700 Fourier transformed infrared spectrometer (Thermo Fisher Scientific Company, USA). The samples were dried before measurement, and the scanning range was 4000–400 cm–1. Pore structure analysis was performed with a Quantachrome Autosorb iQ analyzer using the N2 Brunauer–Emmett–Teller (BET) adsorption method in the partial pressure range of 0.05–0.3. The vacuum freeze-dried samples were degassed at 180 °C for 18 h. The pore size distribution was calculated by the Barrett–Joyner–Halenda (BJH) method.
Thermal Stability of the Prepared Hybrid Gel
The thermal stability of the prepared hybrid gel was determined at temperatures up to 800 °C using a simultaneous TG–DSC measurement (STA449F3) both in the air and nitrogen atmosphere. Samples are lyophilized-dried to remove moisture before use.
Rheological Properties
The rheological properties of the hybrid gel colloids under different ratios of hybrid gel to water were determined by the advanced rheometer (NETZSCH Kinexus lab+) at 25 °C ± 0.1 in this study. The shear rate increased from 0 to 50 s–1, and the area of the thixotropic rings, which consists of the curves of the shear rate and shear stress were measured.
Compressive Strength
The compressive strength of the synthesized hybrid gels was determined in cubical samples (40 mm × 40 mm × 40 mm) after curing using a universal testing machine with a maximum compression capacity of 10 kN and loading rate of 1.0 kN/s.
Determination of Inhibitory Properties
The inhibitory properties of the synthesized hybrid gel on coal spontaneous combustion including the crossing point temperature (CPT) and CO-temperature evolution were determined by the coal spontaneous combustion tendency tester connected with the GC4000A gas-phase chromatography analyzer. The dried hybrid gels were placed in a sufficient quantity of water. 3.75 g of water-saturated hybrid gel and 25 g of coal were mixed thoroughly and the mixture was then placed in a ventilated environment for 1 week to dry naturally. The raw and hybrid gel-treated coal sample was then placed in the reactor, and dry air with the rate of 100 mL/min was allowed to flow through the sample. Set the initial temperature as 30 °C to equilibrate the test system, following which the program-controlled temperature enclosure was set to run at a programmed temperature rise rate of 1 °C/min. During this process, the temperature was continuously recorded, while gas samples at the reactor outlet were analyzed at every 10 °C increase.
Meanwhile, the oxidation behaviors of raw coal samples and hybrid gel-treated coal samples were evaluated by thermo-gravimetric analysis (TGA). The experiments were performed using a simultaneous TG–DSC measurement (STA449F3). Raw coal samples or 15 wt % hybrid gel-treated coal samples were placed on the aluminum crucible, dried air flowed as the reaction gas, and the flow rate of the air purging into the furnace was set as 100 mL/min.
Results and Discussion
Optimization of Preparation Methods
To determine the effects of the parameters on the properties of hybrid gel and find the optimal synthesis conditions, orthogonal arrays design of experiment with the masses of five base materials [bentonite, PAAs, XG, lignocellulose, and Al(OH)3] each at four levels have been performed using gelation time as the key evaluation index. It was found that the use of PAAs and XG with a mass percent concentration above 10 wt % was not possible due to the high prices of these two substances. Moreover, early reports showed that 1.5–2.5 wt % gelling agents such as CMC, MgCl2, AlCl3, or CaCl2 were used in the bentonite-based gel preparation.50,53 For these two reasons, combined with the cheapness and ubiquity of lignocellulose, experimental levels for each factor were established. The factors and levels for orthogonal array design are listed in Table 3.
Table 3. Level of Each Factor for Preparing the Hybrid Gel.
| factors |
|||||
|---|---|---|---|---|---|
| no. | bentonite/g | Al(OH)3/g | PAAs/g | XG/g | lignocellulose/g |
| 1 | 6.00 | 0.10 | 0.20 | 0.05 | 0.50 |
| 2 | 6.00 | 0.30 | 0.30 | 0.10 | 1.00 |
| 3 | 6.00 | 0.50 | 0.40 | 0.15 | 1.50 |
| 4 | 6.00 | 0.70 | 0.50 | 0.20 | 2.00 |
| 5 | 7.00 | 0.10 | 0.30 | 0.20 | 1.50 |
| 6 | 7.00 | 0.30 | 0.20 | 0.15 | 2.00 |
| 7 | 7.00 | 0.50 | 0.50 | 0.10 | 0.50 |
| 8 | 7.00 | 0.70 | 0.40 | 0.05 | 1.00 |
| 9 | 8.00 | 0.10 | 0.40 | 0.10 | 2.00 |
| 10 | 8.00 | 0.30 | 0.50 | 0.05 | 1.50 |
| 11 | 8.00 | 0.50 | 0.20 | 0.20 | 1.00 |
| 12 | 8.00 | 0.70 | 0.30 | 0.15 | 0.50 |
| 13 | 9.00 | 0.10 | 0.50 | 0.15 | 1.00 |
| 14 | 9.00 | 0.30 | 0.40 | 0.20 | 0.50 |
| 15 | 9.00 | 0.50 | 0.30 | 0.05 | 2.00 |
| 16 | 9.00 | 0.70 | 0.20 | 0.10 | 1.50 |
| Gelation Time/m | |||||
| level 1 | 2.20 | 2.58 | 3.53 | 2.55 | 2.85 |
| level 2 | 2.25 | 2.55 | 2.60 | 2.53 | 2.33 |
| level 3 | 2.30 | 2.28 | 2.28 | 2.55 | 2.70 |
| level 4 | 3.45 | 2.80 | 1.80 | 2.58 | 2.33 |
| difference | 1.25 | 0.52 | 1.73 | 0.05 | 0.52 |
According to the analysis of orthogonal experimental design, the relation between factors and gelation time and the contribution of each factor to the gelatinizing time when preparing hybrid gel were seen in Figure 1.
Figure 1.
Relations between gelation time and the factors (a) and contribution of the effect of each factor (b).
It is noted that the masses of PAAs and bentonite are the most effective parameters in the synthesis of the hybrid gel, followed by the addition of gelatinizing agent and lignocellulose, while XG had the weakest effect. It may conclude that the suitable prescription for the synthesis of the bentonite-based hybrid gel is as follows: 8 g of bentonite, 0.5 g of Al(OH)3 (gelatinizing agent), 0.5 g of PAAs, 0.05 g of XG, and 1 g of lignocellulose.
Characterization of Bentonite-Based Hybrid Gel
Morphology Analysis
Based on the optimal prescription, the bentonite-based hybrid gel is synthesized. The morphologies of bentonite and bentonite-based hybrid gel are shown in Figure 2. It can be seen that there are huge compact particles formed by twisting of plates with smooth surfaces in the original bentonite. Whereas, with the addition of organic compounds and gelatinizing agents, the smooth and dense surface of bentonite particles was changed to many slit pores and pleats, similar to the morphology of organics modified montmorillonites reported by Orucoglu54 and Pálková.55 The lamellar structure of montmorillonite exfoliated and formed flakes due to the addition of organic matter. The exfoliated structure and the introduced organic molecules are connected by chemical bonding and results in polymeric matrix formation. In addition, the uniform polymeric matrix of the gel is interlaced with linear tubular structures, which should be formed by linear molecules of lignocellulose and XG inserted into the polyacrylic polymer network structure between bentonite layers. These results indicated that organics were entrapped into the layers of the bentonite. In the figures of EDS spectra of bentonite and hybrid gel (Figure 2), both pristine bentonite and hybrid gel show strong signals for Si, O, and Al, which are the major components of inorganic aluminosilicate gel. There is no C (carbon) signal in bentonite, which is a major component in organic polymer, while in the hybrid gel, the presence of C with 15.36 wt % is ascertained. Therefore, the presence of organic polymer and intercalation of bentonite in the hybrid gel matrix was also substantiated.
Figure 2.
SEM–EDS of bentonite and hybrid gel: (a) bentonite and (b) hybrid gel.
Infrared spectroscopy was used for identifying the formation of a bentonite-based hybrid gel. FTIR spectra of original bentonite, and the prepared bentonite-based hybrid gel are shown in Figure 3. The absorption band at 3631 cm–1 in the spectrum of original bentonite is characteristic of Al–OH stretching vibration of bentonite, and the band at 1043 cm–1 can be ascribed to the antisymmetric stretching vibration of Si–O. The bands around 1650 and 1446 cm–1 are attributed to asymmetry stretching vibration and symmetry stretching vibration of C=O in carboxylate.35,56 Compared to the original bentonite sample, in the spectrum of bentonite-based hybrid gel, the absorption peak of Si–O shifts to a lower wavenumber, and the peak intensity weaken, indicating the formation of a three-dimensional network structure in the hybrid gel. It is also noted that adsorption peaks at 2920 and 2846 cm–1 are observed in the spectrum of hybrid gel, which is characteristic of methyl and methylene groups of XG and lignocellulose. This indicates that XG and lignocellulose were introduced into the hybrid gel successfully. Moreover, after the introduction of organic compounds into bentonite, the absorption band at 3631 cm–1 is merged to 3446 cm–1, and the absorption peak changes from a sharp absorption peak to a broad absorption peak. This change is ascribed to the intermolecular hydrogen bonding between the hydroxyl group of Al–OH and Si–OH in original bentonite and the hydroxyl group in the organic compound, resulting in the association. In addition, the coordination interaction of Al3+ with carboxyl groups in PAAs, XG, and lignocellulose also contributed to the offset of the hydroxyl absorption peak. The coordination interaction that happened between carboxyl groups and Al3+ on the surface of clay has been reported by Nie et al.57 Therefore, in the bentonite-based hybrid gel, coordination interaction of organic polymer and Al3+ on the bentonite surface is proved to exist in the bentonite polymer matrix.
Figure 3.

FTIR spectra of sodium bentonite and the synthesized hybrid gel.
The synthesized hybrid gel was also measured by XRD. Figure 4 showed X-ray intensity profiles obtained from the dry powders of bentonite and the prepared hybrid gel. As seen in the figure, a slight displacement can be observed for the typical diffraction peak of sodium bentonite and bentonite-based hybrid gel. The diffraction peak at 2θ = 7.45° corresponding to the layered structure has shifted to 7.21° after the interaction of organic compounds, and the intensity of the diffraction peak also increased, indicating that the d-value has been expanded from 1.18 to 1.23 nm after the addition of organic polymers, further indicating successful intercalation of organic compounds to the interlayer of bentonite, which is consistent with the result of SEM. At the same time, there is a diffraction peak of 18.27° detected in the hybrid gel, which is the peak of gelatinizing agent Al(OH)3. These results indicated that the organic polymer can enter the interlayer of bentonite and increase the d-space; thus, the crosslinking polymer matrix has been achieved successfully.
Figure 4.

X-ray intensity profiles of dry bentonite and hybrid gel powders.
Pore Structure Analysis
Because the prepared hybrid gel has a porous spongy structure, pore structure analysis of pristine bentonite and the prepared hybrid gel was also conducted and compared. N2 adsorption–desorption isotherms of raw bentonite and the prepared bentonite-based hybrid gel are shown in Figure 5. It is obvious that the adsorption–desorption isotherms exhibit type IV characteristics, indicating that both raw bentonite and the hybrid gel are typical mesoporous materials.58 The hysteresis loop is visible in the regime of mesopore filling and shows typical H3 characteristics attributed to an aggregate of layered structures.59
Figure 5.
N2 adsorption–desorption isotherms of raw bentonite and the prepared hybrid gel.
The BJH model is used to calculate the pore size distributions. Figure 6 showed the pore size distribution of bentonite and the prepared hybrid gel. Although the pore radius of both bentonite and the prepared hybrid gel are at a regime of mesopores, the hybrid gel exhibits a stronger peak intensity, further indicating more pores have been formed in the hybrid gel.
Figure 6.

Pore size distribution of bentonite and bentonite-based hybrid gel.
It also can be seen from pore structure parameters of bentonite and bentonite-based hybrid gel (Table 4) that the BJH pore size of the hybrid gel is slightly less than that of bentonite, whereas both specific surface area and pore volume are significantly increased due to the formation of gel, indicating that the number of pores in the bentonite-based hybrid gel structure was greatly increased, that is to say, there were more pores in the network of the prepared hybrid gel, which was consistent with SEM results.
Table 4. Pore Structure Parameters of Bentonite and Bentonite-Based Hybrid Gel.
| samples | BET (m2/g) | BJH pore size (nm) | total pore volume (cm3/g) |
|---|---|---|---|
| bentonite | 5.064 | 3.932 | 0.0258 |
| bentonite-based hybrid gel | 28.624 | 3.134 | 0.0527 |
Thermal Stability Analysis
Under nitrogen and air atmosphere conditions, TGA of the hybrid gel was conducted with a heating rate of 10 K/min from r.t. to 800 °C to investigate the thermal stability. Figure 7 presents TG curves of hybrid gel heated in nitrogen and air. Similar to the results obtained under oxidative atmosphere, the TG curves both experiences a slight decline during the whole heating process, with residual weights of 82 and 78% in nitrogen and air at the end, respectively. Hybrid gel heated in the air has a more rapid weight loss rate than hybrid gel heated in nitrogen when the temperature is above 100 °C, and the difference in weight loss rates between these two atmospheres is very small as the temperature is below 450 °C, and slightly widen when the temperature is above 450 °C. According to Bors et al.,60 the carbon chains in organic modified bentonite underwent strong oxidation around 230 °C, which could explain why the hybrid gel loses weight faster in air than in nitrogen when the temperature is higher than 100 °C and also supports the result that the organic molecules have been successfully inserted into the interlayer of bentonite. In general, the thermal prepared hybrid gel still retained better thermal stability than ordinary organic gels and is somewhat equal to some inorganic gels.
Figure 7.
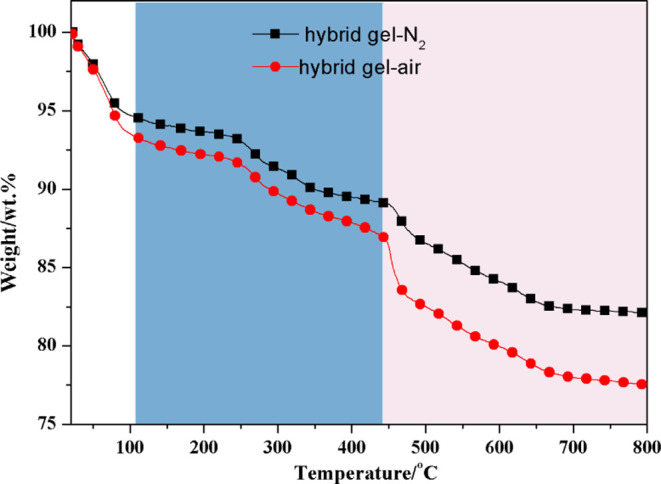
Comparison of TG data collected for hybrid gel heated either in nitrogen or in air.
Swelling Properties and Gelatin Time of the Hybrid Gel
Water Absorption Capacity and Gelatin Time
Water absorption capacity is an important parameter to evaluate the basic performance of hydrogels. The stronger the water absorption capacity of hydrogels is, the more heat is absorbed through water evaporation, which directly affects the effect of oxygen isolation and cooling in actual fire prevention and control work. The gelation time and stability of the gel will also be affected by the water absorption capacity. To investigate the water absorption capacity and gelation time of the prepared hybrid gel, 10 g of dried hybrid gel and a measured amount of water (60 g to 160 g with an increase of 10 g) were mixed and stirred at 600 rpm for 3 min, and then, the timer was started and recorded.
When 10 g of hybrid gel was dispersed in 60 g of water, the colloidal solution gels instantly after stirring, and the gelation time is about 30 s. As shown in Figure 8, the gelation times are less than 1 min as the hybrid gel–water ratios are 1:7, 1:8, and 1:9, and the gelation time is prolonged with the increase of water absorption. As 15 times of water was absorbed by the dry hybrid gel powder, the gel formed successfully 15 min after stopping stirring. However, when the hybrid gel–water ratio reached 1:16, it can still gel, but the colloid still has weak fluidity over 15 min. It can be seen that the prepared hybrid gel has a strong water absorption capacity, and a stable colloid can be formed when the ratio of hybrid gel to water varied from 1:6 to 1:16. Its water absorption has the characteristic of “wide ratio”, and we can only adjust the hybrid gel–water ratio to obtain gel fire-extinguishing material in 30 s to 15 min, thus meeting the needs of fire prevention in different places of coal mine sites.
Figure 8.
Gelation time and stability of hybrid gel mixed with different amounts of water.
Water Retention and Water Absorption Regeneration of the Hybrid Gel
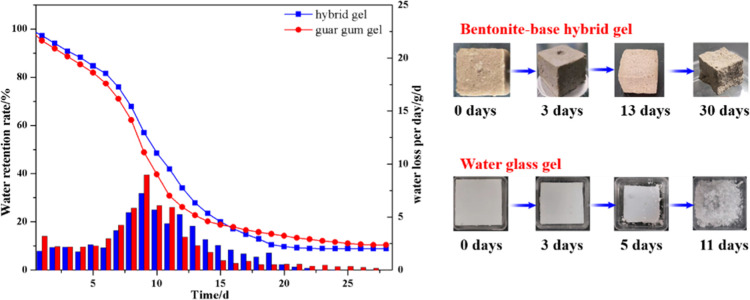
The water retention of the hybrid gel with saturated water has also been measured and compared with guar gum gel which is a common organic gel synthesized by guar gum and sodium borate. Figure 9 shows the water retention of these two samples at room temperature. Similarly, both bentonite-based hybrid gel and guar gum gel lost water slowly, with an average daily loss of 3–5% in the first 6 days. Then, the water loss rates increased with the drying time, and the water retention curves became slightly steeper. Until the 11th day, the water loss rate decreased again and the water retention curve also became flat, but during the first 15 days, the water retention of the hybrid gel is a little bit higher than that of guar gum gel, indicating that the hybrid gel had stronger water retention after forming the composite crosslinking network. As shown in the pore size distribution and the SEM morphology of bentonite-based hybrid gel, more pores are exhibited in the hybrid gel, which provides more swelling room to free water, so more free water can exist in the crosslinking network, and the more water swelled in the network, the longer time needed for complete water loss, which is beneficial to cool down the temperature of coal.
Figure 9.
Water retention and the sample status of the gels.
As we all know, water glass gel widely used in coal mines is an oligomer colloid, and the Si–O bond in the colloid structure is easy to break and leads to powder. As shown in Figure 9, the water glass gel began to pulverize from the 5th day at room temperature. After powder, the gel will no longer be able to plug gaps and isolate coal from oxygen. Thus, it is a prominent and urgent problem to be solved by gel fire extinguishing materials. For the prepared bentonite-based hybrid gel, the polymer network structure is formed by interlayer polymerization of organic molecules between bentonite layers, which enhances the stability of the Si–O–Al bond and makes it difficult to fracture and powder, so the surface of the gel would shrink to form a dense polymeric membrane with the loss of free water.
Rheological Characterization
When gel fire extinguishing materials are used for fire prevention and extinguishing in goaf and stop-production lines, they need to be transported to the ignition point through the pipeline, where certain fluidity comes from the gel shear thinning behavior is needed. Therefore, the shear-dependent rheological properties of the hybrid gel colloid were studied. In this study, the rheological properties of four bentonite-based hybrid gel colloids with different hybrid gel–water ratios (1:10, 1:12, 1:14, and 1:16) were measured (Figure 10). As shown in Figure 10a, the relationship between shear stress and shear rate is not linear, so the prepared hybrid gel colloid does not behave as Newtonian materials. It also can be seen from Figure 10b that the hybrid gel exhibited shear thinning characteristics, and the viscosity of the hybrid gel decreased with the increase in shear rate. The shear thinning behavior was attributed to the chain-expanding structure in the layer of bentonite and increase of entanglements in the main and side chains of XG.61 The results indicated that the shear thinning of the bentonite-based hybrid gel can provide enough fluidity and would be helpful to transport the gel extinguishing material to the ignition point.
Figure 10.
Flow curves of hybrid gel with different amounts of water: (a) shear stress and (b) viscosity.
Compressive Strength Analysis
Despite the bentonite-based hybrid gel colloid having sufficient fluidity, the colloidal solution must gel quickly and has a certain compressive strength, thus filling gaps or covering the surface of coal. The compressive strength of the hybrid gels curing with different days has been investigated. As shown in Figure 11, the hybrid gel curing for 5 days experienced an elastic deformation at low strain and recover to its original shape once unloaded. Moreover, all of the hybrid gels yielded at low strain, followed by a continuous increase of compressive stress which is attributed to an increase in the compression area without damaging the specimen. When the strain reached nearly 80%, the sample was compressed into a cake shape and did not break, so no ultimate compressive strength appeared. The yield stress of the hybrid gels increased from 0.007 to 13.72 MPa, suggesting the enhancement of the compressive strength with the curing time. The reason may be due to the formation of the dense crosslinked polymeric membrane after the loss of water. With excellent compressive strength, the bentonite-based hybrid gel would not break and leak even if it is subjected to strong external forces.
Figure 11.
Compression stress–strain curves for the hybrid gels curing different days: (a) after curing 5, 10, and 15 days and (b) after curing 20 and 30 days.
Analysis of Thermogravimetric Characteristics of Hybrid Gel on Coal
Typical thermogravimetric data obtained from the aerial oxidation measurements for coal samples containing 15 wt % bentonite-based hybrid gel are shown in Figure 12.
Figure 12.
Results of the TGA with raw and hybrid gel-treated coal samples oxidized in the air: (a) oxidation of raw SSP during r.t. −800 °C and (b) oxidation of three coal samples during oxygen uptake.
It can be seen from Figure 12a that the process of coal oxidation can be divided into four parts. The mass of the coal sample decreased with the temperature increase in the first part, which was due to the evaporation of moisture. The second part is ascribed to oxygen chemisorption and the release of volatile material. As observed in the TG curve, there is a slight mass increase in this part, which is attributed to the mass increase of oxygen chemisorption being greater than the release of volatile matter. Followed by the second part, the mass undergoes a dramatic decline as the temperature increases further, and this part is the ignition and combustion process, while the last part is the burnout region. An important feature for oxidation of coal with and without 15 wt % hybrid gel can be found in the TG curves (Figure 12b), that is, the different mass increases occurred in the second oxidation part mentioned above. The results of the mass increase of raw and hybrid gel-treated coal samples are summarized in Table 5.
Table 5. Percentage of Mass Increase at the Second Oxidation Part for Coal Samples.
| samples | percentage of mass increase/% | |
|---|---|---|
| SSP coal | raw SSP | 1.41 |
| hybrid gel-treated SSP | 1.29 | |
| XT coal | raw XT | 1.16 |
| hybrid gel-treated XT | 0.99 | |
| HEXH coal | raw HEXH | 1.71 |
| hybrid gel-treated HEXH | 0.84 |
It is observed that the mass increase is reduced by the introduction of the hybrid gel, which indicates that the hybrid gel can inhibit the oxygen chemisorption well. The ignition temperatures of the raw and hybrid gel treated coal samples can also be obtained from the TG curves, which are shown in Figure 13a. It is evident that the ignition temperature increased with the addition of the hybrid gel; therefore, the hybrid gel appeared to lower the coal oxidation reactivity, thus retarding the ignition temperature.
Figure 13.
Ignition temperatures (a) and CPTs (b) of coal samples with and without the hybrid gel.
Analysis of CPT and CO-temperature Evolution of Hybrid Gel on Coal
The CPT is an important parameter to evaluate the spontaneous combustion tendency of coal. The coal with a lower CPT can be easily ignited and burned. The CPT data obtained from the oxidation measurements of coal samples with and without the hybrid gel are shown in Figure 13b.
Comparing the CPT data of the hybrid gel-treated coal samples with the data of raw coal samples, it is evident that the CPT values increase heavily because of the addition of hybrid gel to the coal samples, indicating that the addition of the hybrid gel inhibits the coal oxidation effectively. As observed from Figure 13b, the order of inhibition on oxidation of the coal samples which was obtained from CPT data can be described as SSP > XT > HEXH. This result can be explained by the metamorphic degree of coal. Among these three coal samples, SSP coal has the lowest metamorphism degree and is most prone to spontaneous combustion. Therefore, the hybrid gel has the most obvious inhibitory effect on SSP.
Various gases, including hydrocarbons, CO2, CO, and water are observed during the coal’s spontaneous combustion. The concentration of CO has a relationship with the reaction rate of coal oxidation, and the concentration of CO generated from coal oxidation has been an important parameter to predict the spontaneous combustion of coal. The slower the oxidation reaction, the less CO is produced. The relationship between the concentration of CO released and temperature for raw and hybrid gel treated coal samples during the temperature programmed process was measured and compared (Figure 14). It is found that the rate of production of CO is slow at temperatures between 30 and 100 °C, which is followed a progressive increase. As the temperature increases further, the concentration of CO increases dramatically with temperature. Moreover, as observed in Figure 16, the concentrations of CO generated by hybrid gel-treated coal samples were much lower than those generated by raw coal samples at the same temperatures, which indicates that the hybrid gel can inhibit coal oxidation effectively.
Figure 14.
Variation of CO concentration with the temperature of coal.
Figure 16.
Formation mechanism of the hybrid gel.
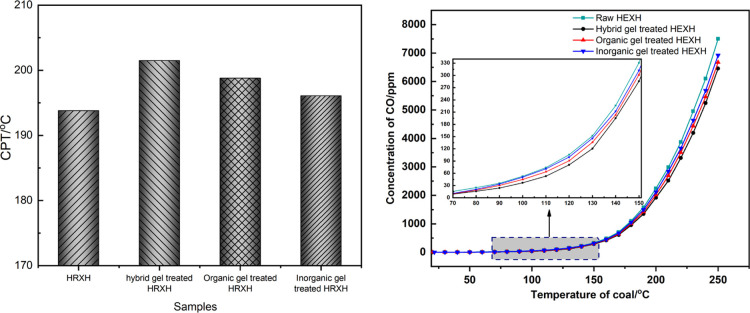
To study the inhibitory efficiency of the hybrid gel further, the CPT data and CO concentration of hybrid gel treated HEXH coal were compared with classical inorganic gel and organic gel-treated HEXH coal. For the inorganic gel, silicic gel which is formed by liquid water glass and sodium bicarbonate is chosen, and organic gel which is synthesized by guar gum and sodium borate is selected. The results are summarized in Figure 15.
Figure 15.
CPT and concentration of CO of different gel-treated HEXH coal.
It can be seen from the figure that the concentration of CO decreased and the CPT increased as the gel was added to HEXH coal, indicating that these gels can prevent the oxidation of coal. Among these three gels, the oxidation of HEXH treated by the hybrid gel produced the least CO and the highest CPT, which indicates that the hybrid gel has the best inhibition effect.
Formation Mechanism of the Hybrid Gel and Its Mechanism of Preventing Coal Oxidation
As described above, the bentonite-based hybrid gel has been synthesized with the advantages of adjustable gelation time, non-cracking, and high compressive strength. Based on the structural characteristics of bentonite, the formation mechanism of the hybrid gel can be inferred as follows. First, Al(OH)3 is introduced into bentonite, a small amount of Al3+ can neutralize the charge with the negatively charged bentonite layer, which reduces or even eliminates the electronegativity of the bentonite crystal layer, weakens the structural force of bentonite lamellar. As analyzed in the results of FTIR spectroscopy, with the introduction of PAA, XG, and lignocellulose, the hydroxyl group in the carboxyl group of PAA reacts with the hydroxide on the bentonite lamellae in a neutralization reaction, causing its insertion into the interlayer of bentonite, while releasing heat, resulting in the widening of the layer spacing and even the peeling of the lamellae, which has been confirmed in XRD and SEM. Afterward, coordination crosslinking polymerization of carboxyl groups and Al3+ occurs to form interlayer polymers with a three-dimensional network structure. According to Papageorgiou et al.62 and Hu et al.,63 the metal–carboxylate complexes can coordinate in different types, such as (I) an ionic or uncoordinated form, (II) unidentate coordination, (III) bidentate chelating coordination, and (IV) bidentate bridging coordination, and the separation of the asymmetric stretching vibration band and symmetric stretching vibration band for C=O in carboxylate of the FTIR spectrum [i.e., Δν = νasym(COO–) – νsym(COO–)] also indicates the structure of metal–carboxylate complexes. In the bentonite-based hybrid gel, Δν is equal to 187 cm–1 which is lower than Δν(COO–)Na reported in literature, indicating that the coordination between carboxyl and Al3+ should be bidentate bridging coordination. Meanwhile, lignocellulose and XG polysaccharide polymer compounds are rich in hydroxyl in the structure, and the hydroxyl groups are crosslinked with polyacrylic acid via hydrogen bond to form a physical crosslinking network, which is homogeneously distributed in the coordination crosslinking network; thus, the dual polymer network structure is finally formed in the layer of bentonite (Figure 16).
The structure of the dual polymer network is composed of two different crosslinking modes: metal coordination and hydrogen bonding. These two types of crosslinking synergy and build the multi-tiered junction, thereby increasing the crosslink density and forming the porous structure. The results of pore size distribution and SEM confirmed the existence of porous structure. This dual network structure has strong stability, which not only supports the layer spacing of bentonite but also enhances the stability of the Si–O–Al bond, thus ensuring the hybrid gel against cracking. At the same time, the metal coordination is chemical crosslinking, and the hydrogen bond belongs to physical crosslinking, when the bentonite-based hybrid gel is subjected to external forces, the hydrogen bonds first act as sacrificial bonds to dissipate external energy. The energy is dissipated through the deformation or displacement of linear molecules of lignocellulose and XG. After the external forces are removed, the hydrogen crosslinked bonds can be formed again. Therefore, the yield strength and compressive strength of bentonite-based hybrid gel are improved, and the gel has good toughness, which is shown in the analysis of the compressive strength of the hybrid gel.
Conclusions
The hybrid gel formed by bentonite, PAAs, Al(OH)3, lignocellulose, and XG was prepared by dual crosslinking in the layer of bentonite, which contains coordination crosslinking and hydrogen bond crosslinking. The synthesized bentonite-based hybrid gel exhibited an improved porous structure compared with bentonite, leading to wide ratios of water adsorption (1:6 to 1:16), excellent water retention, and adjusting gelation time. The hybrid gel also possesses shear thinning characteristics and strong compressive strength, and the yield stress reaches up to 13 MPa. With the enhancement of the stability of Si–O–Al inside the network of the gel, the prepared bentonite-based hybrid gel overcomes the shortcomings of cracking and powdering of existing inorganic gel. With these improved characteristics in hand, the resulting bentonite-based hybrid gel can be used as fire extinguishing gel material in coal mines. The effect of the bentonite-based hybrid gel on coal oxidation measured by TG has shown that the addition of the hybrid gel leads to a significant reduction of mass increase during the period of oxygen chemisorption, thus increasing ignition temperatures. The CPTs and CO-temperature evolution of hybrid gel on coal oxidation show that the oxidation of coal treated by bentonite-based hybrid gel has the highest CPT and produces the lowest concentration of CO. Thus, the present work demonstrates the feasibility of intercalating PAAs and lignocellulose/XG into bentonite to prepare bentonite-based hybrid gel fire-extinguishing materials, and the bentonite-based hybrid gel prevents the coal oxidation effectively.
Acknowledgments
We are grateful for the funding from the National Natural Science Foundation of China (nos 52074286 and 52174213) and funding from the Priority Academic Program Development of Jiangsu Higher Education Institutions.
The authors declare no competing financial interest.
References
- Onifade M.; Genc B. Spontaneous combustion of coals and coal-shales. Int. J. Min. Sci. Technol. 2018, 28, 933–940. 10.1016/j.ijmst.2018.05.013. [DOI] [Google Scholar]
- Takaishvili L.; Sokolov A.; Sokolov B.; Sodovyn B. Development prospects of coal-fired power industry in Mongolia. Proc. Irkutsk State Tech. Univ. 2019, 23, 137–147. 10.21285/1814-3520-2019-1-137-147. [DOI] [Google Scholar]
- Stracher G. B.; Taylor T. P. Coal fires burning out of control around the world: thermodynamic recipe for environmental catastrophe. Int. J. Coal Geol. 2004, 59, 7–17. 10.1016/j.coal.2003.03.002. [DOI] [Google Scholar]
- Carras J. N.; Day S. J.; Saghafi A.; Williams D. J. Greenhouse gas emissions from low-temperature oxidation and spontaneous combustion at open-cut coal mines in Australia. Int. J. Coal Geol. 2009, 78, 161–168. 10.1016/j.coal.2008.12.001. [DOI] [Google Scholar]
- Chen G.; Ma X.; Lin M.; Lin Y.; Yu Z. Study on thermochemical kinetic characteristics and interaction during low temperature oxidation of blended coals. J. Energy Inst. 2015, 88, 221–228. 10.1016/j.joei.2014.09.007. [DOI] [Google Scholar]
- Onifade M.; Genc B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol. 2020, 30, 303–311. 10.1016/j.ijmst.2020.03.001. [DOI] [Google Scholar]
- Wang H. D.; Bogdan Z.; Kennedy E. M. Thermal decomposition of solid oxygenated complexes formed by coal oxidation at low temperatures. Fuel 2002, 81, 1913–1923. 10.1016/s0016-2361(02)00122-9. [DOI] [Google Scholar]
- Wang J.; Zhang J.; Zhu K.; Zhou L. Anatomy of explosives spontaneous combustion accidents in the Chinese underground coal mine: Causes and prevention. Process Saf. Prog. 2016, 35, 221–227. 10.1002/prs.11816. [DOI] [Google Scholar]
- Küçük A.; Kadıoğlu Y.; Gülaboğlu M. Ş. A study of spontaneous combustion characteristics of a turkish lignite: particle size, moisture of coal, humidity of air. Combust. Flame 2003, 133, 255–261. 10.1016/S0010-2180(02)00553-9. [DOI] [Google Scholar]
- Wang H.; Dlugogorski B. Z.; Kennedy E. M. Coal oxidation at low temperatures: oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog. Energy Combust. Sci. 2003, 29, 487–513. 10.1016/s0360-1285(03)00042-x. [DOI] [Google Scholar]
- Wang H. D.; Dlugogorski Z.; Kennedy E. M. Pathways for Production of CO2 and CO in Low-Temperature Oxidation of Coal. Energy Fuels 2003, 17, 150–158. 10.1021/ef020095l. [DOI] [Google Scholar]
- Fan S.; Wen H.; Zhang D.; Yu Z. Experimental Research on the Performance of the Macromolecule Colloid Fire-Extinguishing Material for Coal Seam Spontaneous Combustion. Adv. Mater. Sci. Eng. 2019, 2019, 1–10. 10.1155/2019/6940985. [DOI] [Google Scholar]
- Song Z.; Kuenzer C. Coal fires in China over the last decade: A comprehensive review. Int. J. Coal Geol. 2014, 133, 72–99. 10.1016/j.coal.2014.09.004. [DOI] [Google Scholar]
- Vinogradov A. V.; Kuprin D. S.; Abduragimov I. M.; Kuprin G. N.; Serebriyakov E.; Vinogradov V. V. Silica Foams for Fire Prevention and Firefighting. ACS Appl. Mater. Interfaces 2016, 8, 294–301. 10.1021/acsami.5b08653. [DOI] [PubMed] [Google Scholar]
- Zhai X.; Deng J.. A New Fire-fighting Gel for the Outcrop Fires of the Coal Seam. Progress in Safety Management Research and Practice, 2009.
- Huang Z.; Sun C.; Gao Y.; Ji Y.; Wang H.; Zhang Y.; Yang R. R&D of colloid components of composite material for fire prevention and extinguishing and an investigation of its performance. Process Saf. Environ. 2018, 113, 357–368. 10.1016/j.psep.2017.11.004. [DOI] [Google Scholar]
- Lu W.; Zhang X.; Yuan Y.; Qi G.; Hu X.; Li J.; Liang Y.; Guo B. Study on the characteristics and mechanism of a new type of antioxidant gel foam for coal spontaneous combustion prevention. Colloids Surf., A 2021, 628, 127254. 10.1016/j.colsurfa.2021.127254. [DOI] [Google Scholar]
- Yang F. Q.; Wu C.; Hu H. H.. Fire-extinguishing techniques research on spontaneous combustion of a sulfide iron ore dump in mining stope. Progress in Safety Science and Technology; Science Press, 2008. [Google Scholar]
- Zhou F.; Ren W.; Wang D.; Song T.; Li X.; Zhang Y. Application of three-phase foam to fight an extraordinarily serious coal mine fire. Int. J. Coal Geol. 2006, 67, 95–100. 10.1016/j.coal.2005.09.006. [DOI] [Google Scholar]
- Liu Y. F.; Liu Q.; Long J. F.; Yi F. L.; Li Y. Q.; Lei X. H.; Huang P.; Du B.; Hu N.; Fu S. Y. Bioinspired Color-Changeable Organogel Tactile Sensor with Excellent Overall Performance. ACS Appl. Mater. Interfaces 2020, 12, 49866–49875. 10.1021/acsami.0c12811. [DOI] [PubMed] [Google Scholar]
- Lv J.; Yao X.; Zheng Y.; Wang J.; Jiang L. Antiadhesion Organogel Materials: From Liquid to Solid. Adv. Mater. 2017, 29, 1703032–1703039. 10.1002/adma.201703032. [DOI] [PubMed] [Google Scholar]
- Ma M.; Luan T.; Xing P.; Li Z.; Chu X.; Hao A. Low molecular weight organic compound gel based on cyclodextrin. Prog. Chem. 2019, 31, 225–235. 10.7536/PC180611. [DOI] [Google Scholar]
- Murdan S. Organogels in drug delivery. Expet Opin. Drug Deliv. 2005, 2, 489–505. 10.1517/17425247.2.3.489. [DOI] [PubMed] [Google Scholar]
- Sahoo S.; Kumar N.; Bhattacharya C.; Sagiri S. S.; Jain K.; Pal K.; Ray S. S.; Nayak B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2012, 14, 95–108. 10.1163/138577211x555721. [DOI] [Google Scholar]
- Cheng W.; Hu X.; Xie J.; Zhao Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. 10.1016/j.fuel.2017.09.007. [DOI] [Google Scholar]
- Dou G.; Liu J.; Jiang Z.; Jian H.; Zhong X. Preparation and characterization of a lignin based hydrogel inhibitor on coal spontaneous combustion. Fuel 2022, 308, 122074–122084. 10.1016/j.fuel.2021.122074. [DOI] [Google Scholar]
- Huang Z.; Liu X.; Gao Y.; Zhang Y.; Li Z.; Wang H.; Shi X. Experimental study on the compound system of proanthocyanidin and polyethylene glycol to prevent coal spontaneous combustion. Fuel 2019, 254, 115610–115619. 10.1016/j.fuel.2019.06.018. [DOI] [Google Scholar]
- Li S.; Zhou G.; Wang Y.; Jing B.; Qu Y. Synthesis and characteristics of fire extinguishing gel with high water absorption for coal mines. Process Saf. Environ. Prot. 2019, 125, 207–218. 10.1016/j.psep.2019.03.022. [DOI] [Google Scholar]
- Bouras O.; Houari M.; Khalaf H. Using of surfactant modified Fe-pillared bentonite for the removal of pentachlorophenol from aqueous stream. Environ. Technol. 2001, 22, 69–74. 10.1080/09593332208618307. [DOI] [PubMed] [Google Scholar]
- Jung Y.; Son Y. H.; Lee J. K.; Phuoc T. X.; Soong Y.; Chyu M. K. Rheological behavior of clay-nanoparticle hybrid-added bentonite suspensions: specific role of hybrid additives on the gelation of clay-based fluids. ACS Appl. Mater. Interfaces 2011, 3, 3515–3522. 10.1021/am200742b. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Li Y.; Zhang J. Sorption of organobentonites to some organic pollutants in water. Environ. Sci. Technol. 1997, 31, 1407–1410. 10.1021/es960641n. [DOI] [Google Scholar]
- Guan X.; Yuan X.; Zhao Y.; Bai J.; Li Y.; Cao Y.; Chen Y.; Xiong T. Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd (II) and Pb (II) removal. J. Colloid Interface Sci. 2022, 612, 572–583. 10.1016/j.jcis.2021.12.151. [DOI] [PubMed] [Google Scholar]
- Liu X.; Luan S.; Li W. Utilization of waste hemicelluloses lye for superabsorbent hydrogel synthesis. Int. J. Biol. Macromol. 2019, 132, 954–962. 10.1016/j.ijbiomac.2019.04.041. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Ye H.; Zhou J.; Tang G.; Hou Z.; Bai H. Montmorillonite-Enveloped Zeolitic Imidazolate Framework as a Nourishing Oral Nano-Platform for Gastrointestinal Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 49431–49441. 10.1021/acsami.0c15494. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yang L.; Zhang J.; Wang C.; Li Q. Preparation and characterization of chitosan-poly(vinyl alcohol)/bentonite nanocomposites for adsorption of Hg(II) ions. Chem. Eng. J. 2014, 251, 404–412. 10.1016/j.cej.2014.04.089. [DOI] [Google Scholar]
- Gao G.; Du G.; Sun Y.; Fu J. Self-healable, tough, and ultrastretchable nanocomposite hydrogels based on reversible polyacrylamide/montmorillonite adsorption. ACS Appl. Mater. Interfaces 2015, 7, 5029–5037. 10.1021/acsami.5b00704. [DOI] [PubMed] [Google Scholar]
- Starodoubtsev S. G. L.; Khokhlov A. R.; Allegra G.; Famulari A.; Meille S. V. Mechanism of Smectic Arrangement of Montmorillonite and Bentonite Clay Platelets Incorporated in Gels of Poly(Acrylamide) Induced by the Interaction with Cationic Surfactants. Langmuir 2006, 22, 369–374. 10.1021/la0505869. [DOI] [PubMed] [Google Scholar]
- Modak S. K.; Mandal A.; Chakrabarty D. Studies on synthesis and characterization of poly(methyl methacrylate)-bentonite clay composite by emulsion polymerization and simultaneousin situclay incorporation. Polym. Compos. 2013, 34, 32–40. 10.1002/pc.22374. [DOI] [Google Scholar]
- Fan Y. J.; Zhao Y. Y.; Hu X. M.; Wu M. Y.; Xue D. A novel fire prevention and control plastogel to inhibit spontaneous combustion of coal: Its characteristics and engineering applications. Fuel 2020, 263, 116693–116701. 10.1016/j.fuel.2019.116693. [DOI] [Google Scholar]
- Gioia F.; Urciuolo M. The containment of oil spills in unconsolidated granular porous media using xanthan/Cr(III) and xanthan/Al(III) gels. J. Hazard. Mater. 2004, 116, 83–93. 10.1016/j.jhazmat.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Li P.; Li T.; Zeng Y.; Li X.; Jiang X.; Wang Y.; Xie T.; Zhang Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr. Polym. 2016, 151, 684–691. 10.1016/j.carbpol.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Simões B. M.; Cagnin C.; Yamashita F.; Olivato J. B.; Garcia P. S.; de Oliveira S. M.; Eiras Grossmann M. V. Citric acid as crosslinking agent in starch/xanthan gum hydrogels produced by extrusion and thermopressing. LWT--Food Sci. Technol. 2020, 125, 108950–108956. 10.1016/j.lwt.2019.108950. [DOI] [Google Scholar]
- Gu P.; Liu W.; Hou Q.; Ni Y. Lignocellulose-derived hydrogel/aerogel-based flexible quasi-solid-state supercapacitors with high-performance: a review. J. Mater. Chem. A 2021, 9, 14233–14264. 10.1039/d1ta02281d. [DOI] [Google Scholar]
- Himmel M. E.; Ding S. Y.; Johnson D. K.; Adney W. S.; Nimlos M. R.; Brady J. W.; Foust T. D. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Schutyser W.; Renders T.; Van den Bosch S.; Koelewijn S. F.; Beckham G. T.; Sels B. F. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. 10.1039/c7cs00566k. [DOI] [PubMed] [Google Scholar]
- Bian H.; Wei L.; Lin C.; Ma Q.; Dai H.; Zhu J. Y. Lignin-Containing Cellulose Nanofibril-Reinforced Polyvinyl Alcohol Hydrogels. ACS Sustain. Chem. Eng. 2018, 6, 4821–4828. 10.1021/acssuschemeng.7b04172. [DOI] [Google Scholar]
- Dai X.; Wang J.; Teng F.; Shao Z.; Huang X. Zr(IV)-Crosslinked Polyacrylamide/Polyanionic Cellulose Composite Hydrogels with High Strength and Unique Acid Resistance. J. Polym. Sci., Part B: Polym. Phys. 2019, 57, 981–991. 10.1002/polb.24853. [DOI] [Google Scholar]
- Ding Q.; Xu X.; Yue Y.; Mei C.; Huang C.; Jiang S.; Wu Q.; Han J. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. 10.1021/acsami.8b09656. [DOI] [PubMed] [Google Scholar]
- Benna-Zayani M.; Mgaidi A.; Stambouli M.; Kbir-Ariguib N.; Trabelsi-Ayadi M.; Grossiord J. L. Fractal nature of bentonite-water-NaCl gel systems evidenced by viscoelastic properties and model of gels. Appl. Clay Sci. 2009, 46, 260–264. 10.1016/j.clay.2009.08.014. [DOI] [Google Scholar]
- Li J.; Lu J.; Li Y. Carboxylmethylcellulose/bentonite composite gels: Water sorption behavior and controlled release of herbicide. J. Appl. Polym. Sci. 2009, 112, 261–268. 10.1002/app.29416. [DOI] [Google Scholar]
- Santos R. L.; Horta R. B.; Pereira J.; Nunes T. G.; Rocha P.; Colaço R. Alkali activation of a novel calcium-silicate hydraulic binder with CaO/SiO 2 = 1.1. J. Am. Ceram. Soc. 2018, 101, 4158–4170. 10.1111/jace.15554. [DOI] [Google Scholar]
- Coskuner O. Single Ion and Dimerization Studies of the Al(III) Ion in Aqueous Solution. J. Phys. Chem. A 2010, 114, 10981–10987. 10.1021/jp102906c. [DOI] [PubMed] [Google Scholar]
- Rezaei A.; Nooripoor V.; Shahbazi K. Applicability of Fe3O4 nanoparticles for improving rheological and filtration properties of bentonite-water drilling fluids in the presence of sodium, calcium, and magnesium chlorides. J. Pet. Explor. Prod. Technol. 2020, 10, 2453–2464. 10.1007/s13202-020-00920-6. [DOI] [Google Scholar]
- Orucoglu E.; Haciyakupoglu S. Bentonite modification with hexadecylpyridinium and aluminum polyoxy cations and its effectiveness in Se(IV) removal. J. Environ. Manage. 2015, 160, 30–38. 10.1016/j.jenvman.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Pálková H.; Jankovič L.; Zimowska M.; Madejová J. Alterations of the surface and morphology of tetraalkyl-ammonium modified montmorillonites upon acid treatment. J. Colloid Interface Sci. 2011, 363, 213–222. 10.1016/j.jcis.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Yu L.; Wang D.; Tan Y.; Du J.; Xiao Z.; Wu R.; Xu S.; Huang J. Super tough bentonite/SiO 2 -based dual nanocomposite hydrogels using silane as both an intercalator and a crosslinker. Appl. Clay Sci. 2018, 156, 53–60. 10.1016/j.clay.2018.01.026. [DOI] [Google Scholar]
- Nie H.; Liu M.; Zhan F.; Guo M. Factors on the preparation of carboxymethylcellulose hydrogel and its degradation behavior in soil. Carbohydr. Polym. 2004, 58, 185–189. 10.1016/j.carbpol.2004.06.035. [DOI] [Google Scholar]
- Amziane S.; Collet F.. Bio-aggregates Based Building Materials; Springer, 2017. [Google Scholar]
- Bardestani R.; Patience G. S.; Kaliaguine S. Experimental methods in chemical engineering: specific surface area and pore size distribution measurements-BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. 10.1002/cjce.23632. [DOI] [Google Scholar]
- Bors J.; Patzkó A.; Dékány I. Adsorption behavior of radioiodides in hexadecylpyridinium-humate complexes. Appl. Clay Sci. 2001, 19, 27–37. 10.1016/s0169-1317(01)00060-6. [DOI] [Google Scholar]
- Lee J.-S.; Song K.-W. Time-dependent rheological behavior of natural polysaccharide xanthan gum solutions in interrupted shear and step-incremental/reductional shear flow fields. Korea Aust. Rheol. J. 2015, 27, 297–307. 10.1007/s13367-015-0029-5. [DOI] [Google Scholar]
- Papageorgiou S. K.; Kouvelos E. P.; Favvas E. P.; Sapalidis A. A.; Romanos G. E.; Katsaros F. K. Metal-carboxylate interactions in metal-alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. 10.1016/j.carres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Hu W.; Lu L.; Li Z.; Shao L. A facile slow-gel method for bulk Al-doped carboxymethyl cellulose aerogels with excellent flame retardancy. Carbohydr. Polym. 2019, 207, 352–361. 10.1016/j.carbpol.2018.11.089. [DOI] [PubMed] [Google Scholar]