Abstract

Although graphene has exceptional properties, they are not enough to solve the extensive list of pressing world problems. The substitutional doping of graphene using heteroatoms is one of the preferred methods to adjust the physicochemical properties of graphene. Much effort has been made to dope graphene using a single dopant. However, in recent years, substantial efforts have been made to dope graphene using two or more dopants. This review summarizes all the hard work done to synthesize, characterize, and develop new technologies using codoped, tridoped, and quaternary doped graphene. First, I discuss a simple question that has a complicated answer: When can an atom be considered a dopant? Then, I briefly discuss the single atom doped graphene as a starting point for this review’s primary objective: codoped or dual-doped graphene. I extend the discussion to include tridoped and quaternary doped graphene. I review most of the systems that have been synthesized or studied theoretically and the areas in which they have been used to develop new technologies. Finally, I discuss the challenges and prospects that will shape the future of this fascinating field. It will be shown that most of the graphene systems that have been reported involve the use of nitrogen, and much effort is needed to develop codoped graphene systems that do not rely on the stabilizing effects of nitrogen. I expect that this review will contribute to introducing more researchers to this fascinating field and enlarge the list of codoped graphene systems that have been synthesized.
1. Introduction
Humanity is facing a point of no return because if most of the planet’s population does not change their lifestyle, we will unchain the sixth extinction event on Earth. Chemistry alone cannot solve all the problems; it cannot stop wars or end racism or religious conflicts. However, chemistry can help humanity solve many pressing difficulties that urgently need to be circumvented, such as energy production, conversion, and storage, water purification, development of CO2 capture systems, drug design, and food production, to mention just a few. To that end, discovering new materials with tailored properties has become an obsession. Graphene1,2 is one of the rising stars in science. Nonetheless, despite its fantastic natural properties, the pristine form of graphene has limited use. Up to now there is not a flagship product derived from graphene as there is the transistor derived from silicon.
Several methods have been designed to modify graphene: it can be chemically functionalized,3,4 external stress or electrical fields can be applied, and some of the carbon atoms of the graphene framework can be replaced by heteroatoms, a process that introduces an impurity in the system.5−12 The latter will be the focus of this review. Although significant advances have been made in the preparation of single atom doped graphene, and the properties induced by elemental doping are fascinating,5−12 a new research direction has been opened: the introduction of more than one dopant type in the graphene framework. This transformation is known as the codoping of graphene or sometimes as the dual doping of graphene when it exclusively refers to introducing two foreign atoms. Experimental research and theoretical research have demonstrated that the introduction of at least two dopants can dramatically alter the physicochemical properties of graphene. As an example, I mention the adsorption of lithium on mono- and codoped graphene with 2p and 3p elements.13 As we can appreciate in Table 1, the adsorption energies of Li on N and S doped graphene are −0.72 and −1.25 eV, respectively. Interestingly, when the N and S dopants are introduced simultaneously, the adsorption energy of Li becomes −2.99 eV, about 1 eV larger than the sum of the adsorption energies of Li on N and S doped graphene.13 This is not an isolated example of the dramatic effects that codoping can induce, as I will describe in this review.
Table 1. Interaction Energies, Magnetic Moments, Li–X Distances and Charges Determined for XY Dual Doped Graphene Interacting with a Single Lithium Atom, at the VDW-DF/DZP Level of Theory.
| IE (eV) |
|||||
|---|---|---|---|---|---|
| XY | position of Li | XY orthoa | XY parab | dLi–X (Å) XY ortho | Q (μB) XY ortho |
| AlB | below Al | –2.11 | –2.52 | 2.75 | 1.0 |
| AlN | below Al | –2.16 | –2.03 | 2.66 | 1.0 |
| AlO | below Al | –2.27 | –2.23 | 2.83 | 0.0 |
| SiB | below Si | –2.41 | –2.59 | 2.62 | 0.62 |
| SiN | below Si | –2.18 | –2.14 | 2.70 | 0.0 |
| SiO | below Si | –1.97 | –1.90 | 2.77 | 0.0 |
| PB | below P | –1.89 | –1.45 | 2.67 | 0.06 |
| PN | below P | –1.44 | –1.41 | 2.65 | 0.19 |
| PO | below P | –2.30 | –1.34 | 2.73 | 0.0 |
| SB | below S | –1.79 | –1.87 | 2.81 | 0.06 |
| SN | below S and bonded to N | –2.99 | –1.35 | 2.77 | 0.0 |
| SO | below S | –1.84 | –1.38 | 2.74 | 0.0 |
| B | below B-hex | –2.41 | 2.33 | 0.0 | |
| N | below N-hex | –0.72 | 2.34 | 0.0 | |
| graphene | over hex | –1.11 | 2.32 | 0.0 | |
Ortho disposition of dopants.
Para disposition of dopants.
Several examples in the literature that evidence the advantage of introducing two, three, or even four different heteroatoms will be reported below. The present review is organized in the following way: I first discuss in section 2 what can be considered a dopant since there is no consensus in the literature, and then I briefly inform in section 3 which dopants have been introduced in the graphene framework. In section 4, I advocate commenting on the codoped graphene systems that have been studied, and finally, to conclude the review, in section 5 I indicate the challenges that need to be surmounted for the title field to mature. Finally, I apologize for all the articles that have not been included in this review. The literature about doped graphene contains over 1 million articles, thesis, books, and technical reports. Including all of them in a single review would be impossible. Nevertheless, I expect that the nearly 500 works included cover most of the examples available in the literature and contribute to developing this fascinating research area.
2. When can a heteroatom be considered a dopant?
In this review, heteroatom doping of graphene is considered to be attained when at least one carbon atom of graphene is replaced by another element. Although this definition seems straightforward, when reading the literature, it is possible to become confused. For example, several publications claim to have doped graphene with fluorine atoms.14−21 Considering that fluorine has a strong tendency to form only single bonds because it has seven valence electrons, it is not easy to imagine this element embedded in the graphene framework replacing a carbon atom, as it will bind only with one carbon atom. Vineesh et al.14 reported that N and F codoped graphene displays “enhanced electrocatalytic efficiency than the ‘N’ and ‘F’ individually doped graphene” for the oxygen reduction reaction (ORR). In Vineesh’s work,14 nitrogen was introduced by thermal treatment of fluorinated graphene in the presence of melamine. The amounts of N and F introduced were 2 and 4 atom %, respectively. XPS confirmed the presence of nitrogen in several forms—pyrrolic, pyridinic, and graphitic—while in the case of fluorine C–F and F–C–F bonds were confirmed. Along the same line, Jiang et al.15 prepared N and F codoped graphene by thermal treatment of graphene oxide/polyaniline composites and NH4F. It was proposed as an efficient metal-free ORR catalyst in fuel cells. XPS confirmed the presence of N and F. Nitrogen was found in the same configuration discussed above. However, fluorine was proposed to be present in ionic and semi-ionic C–F bonds. Liu et al.16 also reported the preparation of N and F codoped graphene. The method was simple: they fluorinated N-doped reduced graphene oxide using XeF2 at 180 °C. XPS confirmed C–F covalent bonding, and two more peaks corresponding to CF–CFn and CF2 were determined. The material exhibited interesting photoluminescence properties. Although the presented results represented a significant advance over the existing literature, it is hard to accept that the fluorine atoms are dopants. Instead, in my opinion it is fairer to consider fluorine a functional group covalently attached to graphene but not a member of the graphene framework because, once a fluorine atom is added, nothing more can be bonded.
Fluorine is not the only halogen reported to accompany nitrogen or phosphorus dopants. There are reports in the literature of nitrogen and halogen codoped graphene, with particular attention to iodine,22−24 and P–X codoped graphene with X = Cl, Br, and I.25 In general, the amount of iodine is small, below 1%, and XPS is not conclusive on the nature of the iodine dopant. Wu et al.22 determined that, in PI codoped graphene, the high resolution of the XPS signal corresponded to I3 and I5. Thus it is unlikely that iodine was introduced in the graphene framework. Instead, it is more likely to be adsorbed between graphene layers. Nevertheless, it should be remembered that halogens heavier than fluorine have the capability to form multiple bonds and may be able to replace carbon atoms. Finally, it is essential to mention that, despite being adsorbed, halogens can effectively “dope” graphene because they are natural σ-donors and π-electron withdrawers.25 Therefore, despite the nature of the halogen dopant, i.e., adsorbed or embedded, it works as a dopant, but adsorbed halogens will not be covered in this review.
Finally, it is interesting to mention the case of oxygen. First, it is crucial to consider that it is a common practice to obtain graphene sheets by reducing graphene oxide. In some cases, the reduction is not complete, and also during this process, dopants are introduced using a suitable gas. Considering that in some cases oxygen is not 100% eliminated, in practice, two dopants are present—the one whose introduction was pursued using a gas (for example) and oxygen—so many cases of single atom doping are truly codoped graphene systems. Zhan et al.23 observed the O 1s peak when preparing the I and N codoped graphene nanocomposite described above. The codoping with iodine and nitrogen better facilitated oxygen bonding than the single atom doping of nitrogen or iodine.23 Therefore, caution should be taken when doping is claimed because doping may have been achieved by functionalization, adsorption, or substitution. The latter process is the one that I discuss herein. I considered that an atom is a dopant if it is embedded in the graphene framework replacing one or more carbon atoms.
3. Brief summary of single-atom-doped graphene
When replacing a carbon atom with a dopant, it is not surprising to find that, as early as 2009, Panchakarla et al.26 reported the preparation of boron26−28 and nitrogen26,28−35 doped graphene. These two elements present atomic radii of 85 and 65 pm, respectively, very close to the value of carbon, 70 pm. Therefore, the graphene sheet will not suffer excessive stress if these elements are embedded in the graphene framework. The influence of precursors and synthesis conditions on the preparation of N-doped graphene have been established in the milestone investigation by Wang et al.30 as early as 2014. Also, for a detailed review of the different strategies available to synthesize N-doped graphene, we refer the readers to the excellent review by Vesel et al.35 In particular, Table 1 of the latter summarizes the postsynthesis of N-doped graphene while Table 2, of the same reference, provides an overview of the literature on the direct synthesis of N-doped graphene. A slightly different situation is observed for Be-doped graphene. Although this element has only four electrons, theoretical calculations have shown that the Be atom does not lie in the graphene but bulges out of the sheet.36,37 The protrusion is significant; for example, in a 5 × 5 graphene sheet, the Be atom is located 0.78 Å above the carbon atoms. Although this system is stable, it is waiting to be synthesized. It is expected to have outstanding properties that may render it valid as an anode material for lithium ion batteries.37 Moving down in the periodic table, Al-doped graphene was studied for more than 10 years by theoretical materials scientists.38−44 It was not until recently that Al-doped graphene was prepared.45−47 It was the last 3p element used to dope graphene. Ullah et al.46 successfully prepared a large area Al-doped graphene by chemical vapor deposition. Zagler et al.47 showed that the Al dopants were found in 3- and 4-fold coordinated configurations. Occasionally N dopants were found to be embedded in the graphene framework and bonded to Al, as predicted by us.48,49
On the contrary, Si-doped graphene50−56 was prepared earlier. In 2012, Zhou et al.50 reported that Si atoms could be found in mono- and divacancies in the graphene layer. When Si is bonded to three atoms, it prefers sp3 hybridization, but if it is bonded to four atoms, sp2 d-like hybridization is adopted. As observed for Al, nitrogen was sometimes found to replace one of the carbon atoms bonded to the Si impurities, again in agreement with our findings.48,49 Si-doped graphene is expected to have outstanding mechanical,52 electronic,53−55 and optical properties.54,55 Also, I have demonstrated that, when it is present in the graphene layer, it can promote cycloaddition reactions,56 which can be difficult to attain on perfect graphene.57 The main effect of the Si dopant is to reduce the enormous deformation energy required to form a covalent bond with the diene or dienophile. It has the same effect as a functional group58 present before the cycloaddition reaction or the SiC surface in which graphene can grow.59
The doping of graphene with phosphorus60−69 has been developed at least since 2012 when Some et al.60 reported the preparation of P-doped graphene, air-stable n-type field effect transistors. The system consisted of P-doped, double-layered graphene sheets. Subsequent reports by Li et al.61 and Zhang et al.62 also reported the preparation of P-doped graphene in 2013. This system has unique electronic and magnetic properties39,40,53,66,67 for designing metal-free ORR catalysts,61,62 Li ion batteries,62 and NH3 sensors.63 It is important to notice that Susi et al.64 were the first to obtain atomic resolution imaging and electron energy loss spectroscopy evidencing the presence of phosphorus atoms in the graphene lattice.
In 2009, I reported one of the first studies about sulfur-doped graphene and found that this material may have remarkable properties.42,43,70 Three years later, Yang et al.71 reported the preparation of sulfur-doped graphene as an efficient metal-free cathode catalyst for ORR reactions. Similarly, Yang et al.72 also reported the preparation of S-doped graphene based on ultrathin graphene oxide for ORR reactions. Also, in 2012, Rao et al.73 used liquid precursors to prepare S-doped graphene. The synthesis of the latter has been mastered during the last years, and many routes are available to obtain S-doped graphene, even large area sheets.71−76
Going down in the periodic table, I studied graphene doped with the 4p elements Ga, Ge, As, and Se.77 These nanomaterials exhibited outstanding electronic and magnetic properties and high reactivity near the dopant site, which may render them valuable catalysts. In 2018, Tripathi et al.78 implanted germanium in a graphene framework. The authors claimed that 74Ge+ was the heaviest impurity implanted into monolayer graphene at that time. This atom can substitute a single carbon atom, bonding to three neighbors, or be 4-fold coordinated in a divacancy. In their 2012 article about S-doped graphene, Yang et al.71 also claimed to have prepared Se-doped graphene by direct annealing of graphene oxide in the presence of diphenyl diselenide in argon. XPS studies confirmed the presence of Se atoms, and Se content did not change after sonication. Therefore, it was concluded that diphenyl diselenide was not adsorbed. However, further studies are necessary to have a more specific description of Se doping. In 2018, Meng et al.79 prepared Se-doped graphene for high-efficiency triiodide reduction in dye-sensitized solar cells. SEM mappings revealed the presence of C, O, and Se. The distribution of Se was uniform, and it was concluded that Se atoms were incorporated into the graphene framework.
Turning our attention to the 3d metals, Toh et al.80 prepared Mn-, Fe-, Co-, and Ni-doped graphene hybrids, which are helpful in electrocatalysis. Carnevali et al.81 doped epitaxial graphene by direct incorporation of Ni adatoms. Scandium-doped graphene was also reported in 2019 by Wen et al.,82 but nitrogen was necessary as a stabilizer. Again, as observed for Al and Si, the presence of Sc–N was confirmed by XPS. Robertson et al.83 studied the dynamics of Fe atoms in graphene single and double vacancies, as can be appreciated in Figure 1, and as early as 2014, Zhao et al.84 reported the synthesis of a free-standing, single-atom-thick Fe layer in a graphene pore. More recently, Mn-doped graphene85 was prepared with a Mn concentration of 0.04 atom %. Magnesium was unambiguously located in a single vacancy, but the introduction in divacancies and other structures were observed also. Nevertheless, their structural identification is not clear.
Figure 1.

False color aberration-corrected TEM images of (a) an Fe substitutional defect in a graphene monovacancy (Fe@MV) and (b) an Fe interstitial defect occupying a divacancy (Fe@DV). (c) Smoothed aberration-corrected TEM image of the Fe@MV shown in (a). (d) DFT optimization of the Fe@MV structure (the inset shows a side view) and (e) a multislice TEM image simulation of the system. (f–h) Similar to (c)–(e) but for a DFT-optimized Fe@DV. Scale bars denote 0.5 nm. Reproduced from ref (83). Copyright 2013 American Chemical Society.
Heavier elements like gold were implanted by Trentino et al.86 using a two-step implantation process. The new doping method utilizes a two-step low-energy ion implantation technique that, according to the authors, “overcomes the limitation posed by momentum conservation on the mass of the implanted species.” The gold atoms occupy double vacancy sites. Finally, Nb-doped graphene was prepared by Li et al.87 The strategy employed to obtain Nb-self-doped graphene was to prepare it from 2D NbC, only by one step of incomplete chlorination. This proposal is similar to the procedure I suggested to prepare Si-doped graphene. The structure is shown in Figure 2. The partial annealing to the SiC layer may leave some Si atoms in a specific disposition of the dopants.88 Finally, Sofer et al.89 reported the preparation of U- and Th-doped graphene. However, XPS was not conclusive in the nature of the dopants since the energies of the U 4f peaks indicated the presence of the form UO3, U3O8, or uranium carbide.
Figure 2.
Top (a) and side (b) views of the optimized unit cell for 4 × 4 siligraphene on 6H-SiC(0001). Carbon, silicon, and hydrogen atoms in SiC are colored in gray, light blue, and white, respectively. Carbon and silicon atoms in the siligraphene layer are colored pink and orange, respectively.
4. Codoped or dual-doped graphene
4.1. B and N Codoped Graphene
Boron and nitrogen are the most obvious choices to dope graphene because they bracket carbon in the periodic table. The fact that they have smaller and larger electronegativities than carbon makes them very appealing to develop metal-free catalysts, thanks to the charge imbalance induced by their presence. In 2013, Zheng et al.90 reported the preparation of B and N codoped graphene with long-term stability and excellent activity for the ORR. The two-step method prevented the formation of catalytically inactive byproducts such as hexagonal boron nitride and induced a cooperative effect between the dopants. One year later, the solvothermal synthesis of B and N codoped graphene was achieved,91 and various theoretical studies were published highlighting the unique electronic properties of this system.92−98 As discussed in section 3, one of the main applications of doped graphene is as a catalyst for the ORR. B and N codoped graphene is not the exception, and multiple studies demonstrated that it could be an excellent catalyst for the ORR.98−108 Another essential use of B and N codoped graphene is in lithium batteries109−112 and sodium ion batteries.113 Huang et al.109 reported a high capacity of up to 909 mAh g–1 and an excellent discharge capacity after 125 cycles. Three-dimensional B and N codoped graphene has been employed to construct symmetric and asymmetric supercapacitor electrodes.114−116 The work by Kang et al.114 demonstrated that the specific capacitance of 283 F g–1 at 1 A g–1, in alkaline aqueous electrolyte, is more remarkable than those corresponding to N or B monodoped graphene.
In the catalysis area, it has been demonstrated that B and N codoped graphene can be an excellent catalyst for the electrooxidation of formic acid117 methanol118 and the reduction of nitroarenes119 and triiodide.120 The development of graphene based sensors is another field in which B and N codoped graphene has excelled. It has been able to detect aflatoxin B1,121 mercury(II),122,123 fluorine ions,123 hydrogen peroxide,124 cymoxanil,125 and NO2,126 to mention just a few examples of molecules that can be monitored using B and N codoped graphene.
B and N codoped graphene quantum dots have fascinating optical properties.127−130 They can be used in imaging and photothermal therapies.128 In a related area, B and N codoped graphene was used in dye-sensitized solar cells,129,130 as a counter electrode for iodine reduction.130 Finally, it has been shown that codoping with B and N enhances the electromagnetic interference shielding (EMI)131−134 to −42 dB (99.99% of attenuation) at a critical thickness of 1.2 mm.133
4.2. O and N Codoped Graphene
I explained above that I considered an atom a dopant if embedded in the graphene framework replacing one or more carbon atoms. Nevertheless, I have included O and N codoped graphene in this review because plenty of works report preparations and applications of this system.135−146 In 2014, Chen et al.135 reported that N and O codoped carbon hydrogel could be used as substrate-free electrode for highly efficient oxygen evolution reaction.135 The nitrogen atoms were introduced with ammonium hydroxide, but the oxygen atoms were residual oxygen impurities on graphene prepared by a chemical method. Oxygen was present in the forms carboxyl and epoxy, so it is questionable if oxygen is a dopant because COOH and COC groups can be present without replacing carbon atoms. I do not argue against the utility of these groups as catalytic centers for the oxygen evolution reaction, but they should be named functional groups instead of dopants, in my opinion. Again the discussion if oxygen can be considered a dopant emerges. Li et al.138 assembled O and N codoped graphene on hierarchical carbon networks for all-solid-state flexible supercapacitors. XPS studies revealed that 11% of N was pyridinic and 85% was pyrrolic. However, oxygen was located at the edges as hydroxyl groups. Therefore, in this case, the claim that oxygen is a dopant is at least questionable. In our opinion, the material is N-doped graphene functionalized with hydroxyl groups. Supercapacitor electrodes were constructed using N- and O-doped graphene.136−139 Lithium140−143 and potassium143−146 ion batteries based on N and O codoped graphene are available. Excellent potassium storage was achieved. For example, a reversible capacity of 464.9 mAh g–1 at 0.05 A g–1 was reported by Ruan et al.146
4.3. S and N Codoped Graphene
Despite the fact that sulfur does not fit in the graphene plane and bulges out of the sheet,70 the most studied codoped graphene system is sulfur and nitrogen codoped graphene. There are so many articles published about this system147−360 that a review could be written. Including all the works dealing with this system would be an interminable task, so I apologize for the papers not being cited. In 2012, Liang et al.147 reported the one-step synthesis of sulfur and nitrogen codoped mesoporous graphene for the ORR with a synergistically enhanced performance. The ORR activity was comparable to that of the best commercial Pt/C catalysts and better than the activity measured when only one of the dopants was present. The N and S elemental contents were 4.5 and 2.0 atom %, respectively. Nitrogen was present in the typically observed forms of pyridinic, pyrrolic, and graphitic, while sulfur was present in the thiophenic forms of C–S–C. These results are in partial agreement with our investigations on this system. By means of first principles calculations, I studied all codoped graphene systems with one 3p and one 2p element.48,49,148 Our results revealed a notorious preference for the dopants to be located in specific positions. All XY codoped graphene systems (X = B, N, O; Y = Al, Si, P, S) preferred to replace contiguous C atoms (a CC bond) instead of being separated at large distances or in para/meta positions, as can be appreciated in Table 2; the structures are presented in Figure 3. There was only one exception to this empirical rule: Si and B codoped graphene, for which there was a slight preference for the atoms to adopt a para arrangement. The case of S and N codoped graphene is very particular because the atoms replace a CC bond but the dopants are not bonded. In effect, the nitrogen atoms adopt a pyridinic disposition and the sulfur atom protrudes out of the sheet, as shown in Figure 4. Therefore, nitrogen prefers a C–N–C environment and sulfur prefers a C–S–C environment. This particular dopant disposition may explain this system’s unusual properties. For example, adding O2 to S and N codoped graphene is very exothermic, forming an SO2 unit bonded to graphene.148
Table 2. Relative Energies (eV) between the Five Configurations Studied of the Dopants and Heteroatom Bond Distances (Å) at the M06-L/6-31G* and VDW-DF/DZP Levels of Theory.
| ortho M06-L | meta M06-L | para M06-L | para VDW-DF | same latt M06-L | diff latt M06-L | XY distance (Å)a M06-L | |
|---|---|---|---|---|---|---|---|
| AlB | 0.0 | 0.57 | 0.10 | –0.20 | 0.76 | 0.80 | 2.16 |
| AlN | 0.0 | 1.82 | 1.90 | 1.70 | 2.19 | 2.18 | 1.81 |
| AlO | 0.0 | 3.97 | 3.69 | 3.48 | 4.55 | 4.65 | 1.97 |
| SiB | 0.47 | 0.22 | 0.0 | –0.60 | 0.19 | 0.26 | 1.88 |
| SiN | 0.0 | 1.34 | 1.34 | 1.19 | 1.59 | 1.54 | 1.80 |
| SiO | 0.0 | 3.57 | 2.91 | 2.76 | 3.76 | 3.64 | 2.02 |
| PB | 0.0 | 0.35 | 0.04 | –0.05 | 0.60 | 0.67 | 1.80 |
| PN | 0.0 | 1.01 | 0.70 | 0.61 | 1.09 | 1.00 | 1.78 |
| PO | 0.0 | 3.13 | 2.71 | 2.51 | 3.27 | 3.17 | 2.48 |
| SB | 0.0 | 0.86 | 0.82 | 0.67 | 1.25 | 1.34 | 1.82 |
| SN | 0.0 | 1.56 | 1.32 | 1.18 | 1.46 | 1.43 | 2.58 |
| SO | 0.0 | 5.20 | 5.64 | 5.28 | 5.17 | 5.41 | 2.59 |
XY distance corresponds to the 2p and 3p dopants in an ortho arrangement (case a)).
Figure 3.
Substitution sites considered to study the structure of codoped graphene.
Figure 4.

Optimized 6 × 6 unit cell for sulfur and nitrogen dual-doped graphene.
Many methods are capable of producing S and N codoped graphene. It is possible to start from graphene oxide produced via Hummers’ method and use melamine and benzyl disulfide as N and S precursors. A different approach was proposed by Kicinski et al.,155 who copolymerized S- and N-containing heterocyclic aldehydes, whereas Chen et al.156 used methyl blue/montmorillonite composites. A green route to S and N codoped graphene is also available by using Chinese medical herbs, as proposed by Feng et al.157 Along the same line, Wu et al.158 reported that, assisted by supramolecular polymerization, petroleum coke can be converted into S and N codoped graphene. This approach is exciting because it starts from a byproduct of the oil refinery industry. In theory, creating a new synthetic route using a different heterocycle is possible. For example, Zhang et al.159 self-polymerized polydopamide and then reacted the product with cysteine. These are six examples of the methods available to obtain S and N codoped graphene. More synthetic routes can be found in the references cited, but in our opinion, all of them offer advantages and disadvantages, such as the quality of the sheets prepared, their area, and the bonding nature of the dopants. The main problem with the synthetic procedures available is that there is no good structure–activity relationship.
The main application of the prepared S and N codoped graphene materials has been in perhaps the most critical reaction in the industry, the oxygen reduction reaction. Following the first work by Liang et al.147 commented on above, more than 40 papers were published reporting the catalytic power of S and N codoped graphene for the ORR reaction.154,158−201 Most of these works evidence a performance similar to or superior to those of commercial Pt/C catalysts and superior stability. Nevertheless, more work is needed because, to the best of our knowledge, S and N codoped graphene is not the most used catalyst for oxygen reduction/evolution reactions. For a more detailed review of carbon based metal-free ORR catalysts, I refer the reader to the review by Ma et al.202
Another vital reaction that the graphene community has intensively studied is the hydrogen evolution reaction (HER).203−212 Jiang et al.203 used an intelligent approach to synthesize S and N codoped graphene from a mixture of urea, glucose, and phosphoric acid. The high dopant content and porosity conferred a high catalytic activity in the HER reaction with an onset potential of 0.12 V and a Tafel slope of 79 meV/dec, values that are comparable with those of an average metallic catalyst. There have been some improvements in these values. For example, Guruprasad et al.204 reduced the Tafel slope to 47 meV/dec However, to the best of our knowledge, graphene based catalysts have not replaced the ones previously utilized in the industry. In this line, the photocatalytic production of hydrogen from water splitting is crucial for humankind. S and N codoped graphene has been combined with TiO2 to improve the activity under visible light significantly. S and N graphene based materials are generally used as light absorbers combined with TiO2 or similar materials.208,209
The use of S and N codoped graphene as a catalyst is not limited to the ORR and HER reactions. It has been used with great success for catalytic phenol degradation,212,213 nitrogen reduction,214 methanol213−217 and ethanol218 electrooxidation, aerobic oxidation of alcohols219 under visible light irradiation, and sonocatalytic decolorization of methylene blue.220 Another area of utmost importance is organic catalysis. Some works reported using S and N codoped graphene for Sonogashira221 and Heck couplings.222 Although Pd nanoparticles were still necessary, the presence of the graphene material contributed to a more effective use of the expensive Pd catalyst.
There are several examples in which S and N codoped graphene has been employed in photocatalysis.223,224 For example, it can degrade rhodamine B223,224 and methyl orange.225 In the former case, the activity is 3 and 10 times higher than those determined for N-doped graphene and P25 TiO2, respectively.223 Efficient photocatalytic H2O2 production was achieved since the codoped graphene induced a better light absorption and promoted a more significant charge migration.226 Sulfur and nitrogen codoped graphene nanomaterials have broad visible absorption223,224 and superb luminescence properties.226−232 They can be used in bioimaging.233,234 Also, they were reported to have a fluorescence quantum yield 9.3 times higher than that of undoped graphene. Thus, they are promising materials for developing light-emitting devices.228
The development of alkali metal ion batteries is another area that has been invaded by publications that use S and N codoped graphene.235−269 In 2014, Ma et al.153 produced S and N cocoped graphene using a chemical vapor deposition approach. The three-dimensional codoped graphene networks were utilized as anode materials for lithium ion batteries. The capacity was 3525 mAh/g at a current density of 50 mA/g, and the rate capability was as high as 870 mAh/g at 1000 mA/g, with with excellent cycling stability. Following Ma et al.’s153 landmark investigation, several works continued this line of research.235−261 In some cases, metal nanoparticles,235,236 nanocables,237 and nanospheres238 are also combined with S and N doped graphene to improve performance. Although the numbers obtained are impressive, and are in agreement with our theoretical predictions,13 to the best of my knowledge, there is not a commercial lithium ion battery based on S and N codoped graphene. Therefore much work is needed in this area.
The excellent performance observed for lithium batteries prompted researchers to study if the effect was similar for heavier and cheaper alkali metals like Na and K. In effect, superb performances were observed for sodium261−267 and potassium storage.268,269 Recent theoretical calculations that I carried out support these results.152 I demonstrated that, as observed for lithium,13 S and N codoped graphene presents the strongest affinity toward Na and K adsorption.152 The adsorption energies of Na and K are gathered in Tables 3 and 4, respectively, and the structure is giving in Figure 5. The adsorption energies for Na and K were dramatically increased by the presence of the S and N dopants, another system for which I observed comparable adsorption energies was Si and B codoped graphene. Therefore, at least for Na and K, there seems to be another alternative to dope graphene and improve alkali metal storage. Metal–air batteries,270−277 in particular Zn–air ones,270−276 were also constructed using S and N codoped graphene. Geng et al.270 used S and N codoped graphene decorated with CoS nanoparticles as a cathode material for Zn–air batteries. The product could be charged and discharged for 50 cycles at 1.25 mA/cm2, for 50 h with an almost constant discharge voltage of 1.23 V. In sharp contrast, the discharge voltage of the commercial Pt/C catalyst was reduced from 1.36 to 1.20 V. In general, the catalytic performances of the commercial catalyst and the S and N codoped graphene based one were similar, so it is a cheaper alternative. Ganesan et al.271 also claimed that N and S codoped graphene/CoS2 nanoparticles could be a suitable air cathode for Zn–air batteries. Chen et al.272 went one step further and did not use nanoparticles to construct a cathode for Zn–air batteries. Instead, they used S and N codoped graphene microwires and obtained a superior performance. For more details on this topic, I refer the reader to refs (270−277). Finally, vanadium redox flow batteries were built using S and N codoped graphene by Li et al.278 and Daugherty et al.279 An improvement of 85.37% at a current density of 80 mA/cm2 was reported by Li et al.278
Table 3. Sodium Adsorption Energies onto Codoped Graphene 5 × 5 and Circumcoronene.
| dopant | VDW-DF/DZP G5×5a | M06-2X/6-311G* circumcoroneneb | PBE-D2/VASP G5×5 | PBE-D2/6-311G* circumcoronene |
|---|---|---|---|---|
| AlB | –1.58 | –1.81 | –2.44 | –2.10 |
| AlN | –1.55 | –1.98 | –2.44 | –2.05 |
| AlO | –1.63 | –1.64 | –2.43 | –1.99 |
| SiB | –2.13 | –2.90 | –2.72 | –2.94 |
| SiN | –1.63 | –2.11 | –2.42 | –2.09 |
| SiO | –1.42 | –1.34 | –2.21 | –1.62 |
| PB | –1.47 | –1.75 | –2.36 | –1.91 |
| PN | –0.96 | –1.15 | –1.92 | –1.37 |
| PO | –1.71 | –1.97 | –2.51 | –2.18 |
| SB | –1.10 | –1.96 | –2.35 | –2.03 |
| SN | –2.00 | –3.35 | –2.91 | –2.92 |
| SO | –0.97 | –1.30 | –2.13 | –1.49 |
| G5×5 | –0.67 | –0.45 | –1.08 | –0.71 |
Calculations were performed using periodic conditions and a 5 × 5 unit cell of graphene.
Calculations were performed using a graphene flake (circumcoronene).
Table 4. Potassium Adsorption Energies onto Codoped Graphene 5 × 5 and Circumcoronene.
| dopant | VDW-DF/DZP G5×5a | M06-2X/6-311G* circumcoroneneb | PBE-D2/VASP G5×5 | PBE-D2/6-311G* circumcoronene |
|---|---|---|---|---|
| AlB | –1.81 | –2.22 | –2.55 | –2.33 |
| AlN | –1.75 | –2.35 | –2.51 | –2.27 |
| AlO | –1.78 | –1.95 | –2.47 | –2.18 |
| SiB | –2.42 | –3.36 | –3.06 | –3.24 |
| SiN | –1.84 | –2.47 | –2.51 | –2.32 |
| SiO | –1.64 | –1.75 | –2.31 | –1.88 |
| PB | –1.81 | –2.20 | –2.51 | –1.93 |
| PN | –1.40 | –1.57 | –2.05 | –1.65 |
| PO | –1.95 | –2.32 | –2.58 | –1.39 |
| SB | –1.08 | –2.36 | –2.47 | –2.31 |
| SN | –2.42 | –3.64 | –3.03 | –3.05 |
| SO | –1.20 | –1.69 | –2.22 | –1.73 |
| G5×5 | –1.1 | –0.96 | –1.40 | –1.10 |
Calculations were performed using periodic conditions and a 5 × 5 unit cell of graphene.
Calculations were performed using a graphene flake (circumcoronene).
Figure 5.
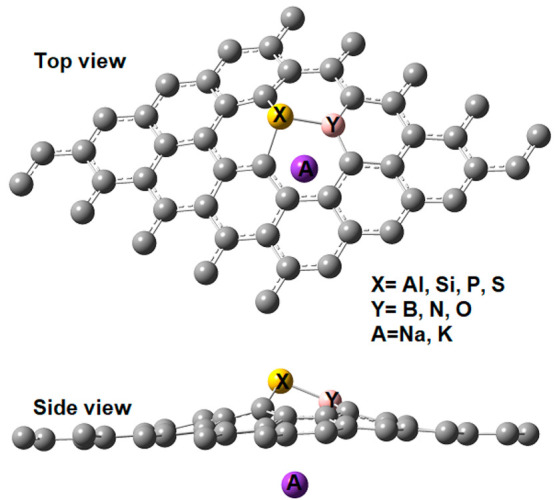
Optimized structure for codoped graphene with an ortho disposition of dopants and an alkali metal atom adsorbed below the 3p dopant.
Symmetric and asymmetric supercapacitor electrodes were created using S and N codoped graphene.280−305 As early as 2015, Xing et al.281 prepared three-dimensional S and N codoped graphene hydrogels using thiocarbohydrazide as a reducing and doping agent. Thanks to the highly porous architecture and presence of dopants, the materials exhibited a high specific capacitance of 141.1 F/g in KOH electrolyte. Their specific capacitance could be maintained at 71.3% even with a discharging current density of 10 A/g. Also, excellent electrochemical stability and reversibility were obtained, with about 90% of retention after 4000 cycles. Even more interesting results were obtained by Tran et al.,283 who prepared a S and N codoped hole defect graphene hydrogel. A maximum energy density of 14.8 Wh/kg and excellent cycle stability were reported. In general, the porous nature of the S and N based materials is crucial for the performance of the supercapacitor.280−305 Comparing the performances of the electrodes prepared is not easy because different conditions are employed. Thus, I refer the reader to refs (280−305) gathered on this topic.
The quest for dye-sensitized solar cells with higher efficiency has also tested if the inclusion of S and N codoped graphene can be useful.306−315 The advantages of codoping as compared with the monodoping when preparing dye-sensitized solar cells (DSSCs) were also confirmed by Luo et al.306 A power conversion efficiency (PCE) of 4.23% was obtained for sulfur reduced graphene oxide, while for sulfur and nitrogen reduced graphene oxide the value increased to 4.73%. In 2015, Xu et al.307 built bifacial DSSCs capable of utilizing incidental light from the rear and front sides that relied on transparent S and N codoped graphene as counter electrodes. A drastically enhanced power conversion efficiency was obtained for both front and rear illumination. Kannan et al.311 reported a PCE as high as 7.42% when S and N codoped graphene was utilized as a counter electrode. The latter value is comparable to the one determined for the Pt counter electrodes, namely 7.42%. The high catalytic activity of the codoped graphene electrode was attributed to the difference in electronegativity between S and N as well as to the structural distortions caused by the presence of the sulfur atom in the graphene framework. Theoretical investigations have also proven the utility of the codoping of graphene in this area. Liu et al.312 performed first principles calculations to show that the synergistic effect between the S and N dopants resulted in a much better charge transfer between the substrate and the adsorbed I2 on the surface of S and N codoped graphene. Therefore, a much better efficiency would be obtained in DSSCs that employ codoped graphene.
S and N codoped graphene based sensors have been extensively studied in the literature.316−347 S and N codoped graphene was used by Li et al.316 as a support for CuO nanoparticles to promote electrocatalytic glucose oxidation. A low detection limit of 0.07 μM was reported by Li et al.316 and a detection limit of 0.5 μM was reported by Tian et al.318 using S and N codoped graphene prepared with a one-step and cost-effective microwave assisted solvothermal method. Masteri-Farahani et al.319 developed a fluorescence based sensor to detect glucose. They combined boronic acid with the codoped graphene sheets to obtain a lower detection limit of 5.5 μM. The codoped graphene based new sensor is much cheaper because it reduces the use of the expensive boronic acid. In the biological area, S and N codoped graphene based sensors have been constructed for l-cysteine,320 hydroquinone/catechol,321 cyanide,322 an immunosensor for cardiac troponin I,323 okadaic acid,324 an immunosensor for cardiac troponin,325 ascorbic acid,326,327 and dopamine,327,328 to mention just a few examples. In the same vein, pesticides329 and toxic pollutants330 can be detected. The sensor array built by Zhu et al.329 was able to discriminate among five pesticides: lactofen, fluoroxypyr-meptyl, bensulfuron-methyl, fomesafen, and diafenthiuron. In this case, there was a practical application of the sensor array as it was validated by discriminating pesticides in soil. In food samples, ethion331 and nitrites332 are easily monitored using the fluorescence properties of S and N codoped graphene quantum dots. Martins et al.333 used glassy carbon electrodes modified with S and N codoped graphene quantum dots for monitoring multivitamins. The detection limits for vitamins B2, B6, and B12 were 0.30, 30.1, and 0.32 nmol/L. The sensor was effectively implemented in the quantification of vitamins in classic and fruit based energy drinks. Simpler molecules like water334 and ammonia335 can be sensed using S and N graphene quantum dots. Jlassi et al.334 constructed a S and N graphene carbon dot based impedance sensor with good sensitivity for water and excellent response and recovery times of 15 s and 55 s, respectively. The detection of explosives is a very sensitive topic that needs constant updates. Mondal et al.336 observed a remarkable increase in fluorescence quenching effect using a micromolar solution of 2,4,6-trinitrophenol (TNP). The detection limit was as low as 19.05 ppb. Zhang et al.337 used an electrochemical sensing platform using S and N codoped graphene nanoribbons that showed a highly sensitive and selective response to trinitrotoluene (TNT) with a wide linear range from 0.0008 to 5.1 ppm and a detection limit as low as 0.1 ppb. Finally, S and N codoped graphene has been employed to detect a variety of metal and nonmetal atoms. For example, the fluorescence detection of Co(II) ions in water was reported by Boonta et al.,338 while the successful detection of Hg2+ ions is well documented.339−342 In general, the detection is related to fluorescence quenching after interacting with the metal ion. Gu et al.340 demonstrated that the sensitivity of S and N graphene carbon dots was 4.23 times higher than that of N-doped counterparts. The sensor was sensitive enough to ensure that drinking water had less than 10 ppb Hg2+ ions. In addition to this, a report by Tian et al.344 revealed that Hg(II) ultraselectively separated from Pb(II) and Cu(II). A high removal ability above 99% was obtained. The list of elements that can be detected in quite long, but it includes Fe3+, with a low detection limit of 0.07 μM,342,343 Pb(II)345 with a detection limit as low as 0.29 nM, iodide341−346 with a detection limit of 4.23 nM, and Cu2+ and Ag+ with detection limits of 250 and 50 nM, respectively.347 Interestingly, the sensor constructed by Zhang et al.341 was an off–on sensor that had the chemiluminescence quenched by iodide and recovered by mercury ions.
We have listed a few areas in which S and N codoped graphene has made an impact, but I limit them to maintain a balance in this review. There are others such as CO2 capture,348 removal of organic dyes,349−352 design of efficient electromagnetic wave absorbers,353−356 piezocatalysis,357,358 bioelectricity production,359 and synthesis of high performance polymer nanocomposites.360
4.4. P and N Codoped Graphene
It is not a surprise that another of the most studied cocoped graphene systems also contains nitrogen, but in this case it is chaperoned by a pnictogen: phosphorus.361−420 In 2014, Zheng et al.361 reported the synthesis of P and N codoped graphene and its use as nonmetal catalyst for the hydrogen evolution reaction. The performance was much better than those corresponding to monodoped graphene and comparable to some commercial catalysts. Two approaches were employed to synthesize this material. In the first approach, a single-step method, graphene oxide was annealed in the presence of melamine and triphenylphosphine, while in the second approach, which involved two steps, first P-doped graphene was obtained and the nitrogen was incorporated. K-edge NEXAFS and XPS studies indicated that nitrogen was present in pyridinic, graphitic, and pyrrolic forms whereas phosphorus was bonded to carbon or oxygen. The presence of P–N bonds was excluded. This result is in contrast to experimental studies that indicated the presence of AlN bonds in Al-doped graphene47 and SiN bonds in Si-doped graphene.51 Also it is in disagreement with our investigations which indicated that the P and N dopants prefer to form PN in P and N codoped graphene. In effect, the ortho disposition of the dopants is 1.01 and 0.70 eV more stable than the meta and para ones, respectively.48,49 A few months later, Xue et al.362 prepared P and N codoped graphene with air-stable n-type characteristics. Phosphorus played a critical role in achieving improved charge donation. The material was synthesized over a Cu surface and using phosphonitrilic chlorine trimer as a P and N source. A different approach to prepare P and N codoped graphene was proposed by Li et al.363 The method was facile and cost-effective; they pyrolyzed a dried hydrogel composed of graphene oxide, polyaniline, and phytic acid. These are three examples of how P and N codoped graphene can be synthesized. Since there is not a standard method to obtain it, I refer the readers to the references included, as most of them report a unique method.361−422 For example, Ananthanarayanan et al.364 used adenosine triphosphate and Guo et al.365 used hypophosphorous acid as a P source to dope graphene oxide.
In line with our discussion about S and N codoped graphene, the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) were the subject of several investigations that tested the viability of using P and N codoped graphene as a catalyst.363,365−373 Li et al.’s363 study was one of the first to not only prepare the material but also to use P and N codoped graphene as a catalyst for ORR and OER reactions. The authors claimed that up to 2015 it was the best nonmetal bifunctional electrocatalyst reported for the latter reaction, with a potential gap of 0.71 V between the OER potential, at a current density of 10 mA/cm2, and the ORR potential, at a current density of −3 mA/cm2. A similar ORR and OER overpotential of 705 mV was reported by Chai et al.366 using P and N codoped graphene prepared via a one-pot hydrothermal method utilizing graphene oxide, ammonium dihydrogen phosphate, and cyanamide as precursors. The P sites were very active and could be oxidized, becoming nonactive for catalysts. However, it was pointed out by Chai et al.366 that if P is oxidized and bounded to an N codopant it stabilizes the graphitic N and increases the reactivity of the neighboring carbon atoms. For this reason the synthetic conditions were tuned to increase the number of P–N bonds, in line with our previous studies.48,49 P and N codoped graphene can be synthesized using green chemistry. Cheng et al.367 used low-cost inorganic fertilizers and graphene oxide as precursors to obtain the said nanomaterial. The material presented super electrocatalytic activity and methanol tolerance. In particular, the electrochemical stability was better than those of commercial Pt/C catalysts. Molina-García et al.368 combined codoped graphene and perovskites to build a catalyst for the ORR reaction. Among the codoped graphenes tested, S/N, P/N, and B/N were employed. The lowest overpotential was obtained when perovskites were combined with P and N codoped graphene. Further experimental369−372 and theoretical373 studies also corroborated the excellent performance of P and N codoped graphene as a catalyst for the ORR.
As mentioned above, the hydrogen evolution reaction has also been studied using P and N codoped graphene. However, it has received less attention than the ORR. Zheng et al.361 demonstrated that the HER activity of P and N codoped graphene is comparable to those of metal catalysts such as gold, molybdenum, and Mo/Ni alloy. However, it is less effective than nanostructured MoS2/WS2. It was speculated that, via nanostructure engineering, the graphene based catalysts have the potential to replace all the commercial ones for the HER. Some years later, Hung et al.374 devised a procedure to dope graphene with P and N, paying particular attention to obtaining better crystallinity, conductivity, and elemental functionalities to obtain improved catalytic performance. The amount of P doping was as high as 6 atom %, and the electrode displayed excellent catalytic activity with an increase of 142% in sp2 domain size and enormous lowering in overpotential and the Tafel slope, namely 78%. The efficiency of the catalyst was 25% better than that of the MoSx one. An et al.375 prepared a stable catalyst composed of a Co/Ru alloy and P and N codoped graphene. The catalyst exhibited high activity, a low overpotential of 52 mV at 10 mA/cm2, and a Tafel slope of 38 mV/dec.
P and N codoped graphene has been used for methanol electrooxidation. Chen et al.376 decorated the doped graphene sheets with Pd nanoparticles. The nanomaterial delivered a catalytic current density of 11.9 A/cm2 in 1 M KOH with 1 M CH3OH at −0.2 V after a test duration of 3600 s. The aerobic oxidation of alcohols was achieved using Co porphyrins supported on P and N codoped graphene.377 The catalyst converted 92% of the sample and exhibited a high selectivity of 86%, better than most reported photocatalysts. Among other processes that P and N codoped graphene can catalyze, I can highlight the work by Xi et al.,378 which reported the reduction of nitroarenes or the good performance of P and N codoped graphene as a cathode electrode catalyst in microbial fuel cells.379
In the biological field, P and N codoped graphene has been used for cellular imaging.364,380,381 Ananthanarayanan et al.364 used P and N codoped graphene quantum dots that exhibited high photostability, strong two-photon upconversion, and small molecular weight for real-time tracking of transferring in live cells. Gong et al.380 also used the doped carbon dots for monitoring because of their exceptional fluorescent properties. The material was also utilized for doxorubicin delivery because the effectivity of the drug was much better when combined with the carbon material. Liu et al.381 were able to use similar carbon dots for sensitive and selective detection of nitrite in live cells. Finally, Shumba and Nyokong382 modified P and N codoped graphene with Co(II) phthalocyanine to detect H2O2; the sensitivity was 12 mA/M and the detection limit was 1.21 nM.
A topic that always is fueled by graphene doped with heteroatoms is that of rechargeable batteries. P and N codoped graphene was successfully used in lithium,383−390 sodium,391−394 potassium,395 and Zn–air batteries.396−398 In 2015, Gu et al.383 prepared P and N codoped graphene using graphene oxide, triphenylphosphine, and ammonia with polytetrafluoroethylene as a binder. They constructed a membrane which was used as blocking layer to conductively confine polysulfides in the cathodes of lithium–sulfur batteries. The performance of the new battery was remarkable and significantly better than the ones prepared using monodoped graphene. The initial capacity was 1158.3 mAh/g at a current density of 1 C. The cycling stability was reasonable, decaying 0.09% per cycle. Wu et al.384 were able to suppress polysulfide dissolution by physical confinement and chemical interaction; the assembled Li–S batteries had an initial discharge capacity of 1446 mAh/g at a current density of 0.1 C. The capacity decay was extremely low: 0.034% per cycle. Other reports by Zhou et al.,385 Zeng et al.,386 and Zhang et al.387 also utilized P and N codoped graphene to solve the shuttle effect of polysulfides and volume expansion of sulfur that seriously affect performance. A slightly different approach was chosen by Muhammad et al.,388 who prepared P and N codoped graphene microspheres embedded with core–shell CoP@C and MoP@C nanoparticles. The interior carbon shell limited volume evolution and prevented nanoparticle aggregation. As a consequence, excellent lithium storage was achieved.
In an attempt to use sodium instead of lithium, Li et al.391 constructed carbonaceous anodes for sodium batteries. P and N codoped graphene was obtained via low temperature phosphidation of NH2 rich graphene precursor. A large reversible capacity of 330 mAh/g at 50 mA/g was measured. Studies by Wang et al.,392 Qin et al.,393 and Wu et al.394 were also devoted to producing sodium battery anodes, with great performances. Potassium ion batteries were prepared by Gao et al.395 using P and N codoped graphene aerogels with a specific capacity of 507 mAh/g at 100 mA/g. Zn–air batteries were the focus of several studies that used P and N codoped graphene as an efficient electrocatalyst.396−398 The 3D P and N codoped holey graphene foam presented excellent activity, showing a half-wave potential of 0.865 V in alkaline electrolytes. The design of supercapacitors is another field that experienced important innovations thanks to the use of P and N codoped graphene.399−413 Wen et al.399 synthesized P and N codoped graphene monoliths by a facile hydrothermal method that used melamine phosphate as a single precursor. Excellent capacitive performance was obtained. Supercapacitors with an energy density of 8.2 Wh/kg were produced by Xia et al.400 when P and N codoped graphene with a high surface area and hierarchical pore structure was utilized. It was prepared by prefunctionalization of graphene and subsequent one-step ammonia phosphate activation. A high specific capacitance of 219 F/g at 0.25 A/g was obtained. Hierarchical porous P and N codoped graphene was also utilized by Zhao et al.;401 the specific capacitance obtained was 204.4 F/g at 0.2 A/g. In this case, the codoped graphene was produced using nitrogen-containing biomass derived compounds in conjunction with phosphoric acid treatment. The specific capacity retained 97% after 2000 cycles. For more examples about this application of P and N codoped graphene, I refer the readers to refs (399−419). In all of them, the advantages of codoping are demonstrated. Finally, it has been postulated that P and N codoped graphene can be used as a flame retardant and smoke suppressant,414−416 CO oxidator,417 O2 adsorbent,418 electronic transport material,419 and oxidation retardant of reduced graphene oxide.420
4.5. Codoped Graphene with Other Main Group Elements
The possibilities to construct codoped graphene systems are almost unlimited as there are plenty of possibilities. In this section I mention some of the systems prepared or studied theoretically. P and B codoped graphene was prepared this year by carbonizing in an Ar atmosphere a cellulose/phosphoric acid supramolecular collosol. Then sodium tetraborate decahydrate was used to adjust the B content, as indicated by Meng et al.421 The nanomaterial presented an outstanding catalytic performance for benzyl alcohol oxidation. As early as 2015, I studied theoretically all 3p/2p codoped graphene systems, finding that P and B codoped graphene was very particular because it was one the few examples for which the stability of the ortho and para dispositions of the dopants were almost equally stable. In fact the ortho configuration, i.e., P and B replacing a CC bond, was more stable by 0.04 eV at the M06-L/6-31G* level.48 The system is nonmagnetic and a semiconductor with a band gap of 0.2 eV at a 2 atom % doping. In a following article, I showed that when a B dopant is added to P-doped graphene, the effective masses of holes and electrons decrease.
Some studies reported the synthesis of P and O codoped graphene. I discussed above if oxygen can be considered a dopant. In this section I recall that any P-doped graphene produced from graphene oxide will have some amount of residual oxygen, which may be comparable to the levels of doping attained for P-doped graphene.60−69 Ma et al.422 reported the preparation of P and O codoped graphene with exceptional properties as anode materials for potassium ion batteries. The authors prepared the codoped graphene by thermal annealing of graphene oxide with triphenylphosphine. The material had ultralong cycling stability and a capacity of 474 mAh/g at 50 mA/g.
In 2016, Yu et al.423 utilized P and S codoped hierarchically porous graphene aerogels for enhancing supercapacitor performance. The material was prepared by heating graphene oxide prepared by the modified Hummers method, thioglycoic acid, and pythic acid. The doping level was 5.8 and 4.6 atom % for S and P, respectively. XPS revealed the presence of S–C and P–C bonds. The specific capacitance was 438 F/g at 19 mV/s, greater than the ones measured for the monodoped counterparts. Patel et al.424 prepared P and S codoped graphitic carbons for aerobic oxidation reactions. Further studies indicated that P and S codoped graphene can be a good catalyst of the HER425 and methanol electrooxidation426 and improved capacitance.427−429
Sulfur and boron doped graphene was synthesized and combined with Au@Pt nanorods. It was utilized as a immunosensing platform for the electrochemical determination of aflatoxin.430 The codoped graphene was prepared using a microwave-assisted hydrothermal approach. Boron trioxide and sodium sulfide were used as heteroatom sources. XPS indicated the presence of C–B and C–S bonds. Theoretical studies characterized SBe,431 SiN,432 NAl,433 and SiP434 codoped graphene, materials which presented outstanding electronic, NO2 sensing, optical, and catalytic properties, respectively. I note that Al and N codoped graphene has not been synthesized, but when Al-doped graphene was reported, Al–N bonds were observed.46,47 A facile synthesis for halogen (Cl, Br, and I) and nitrogen codoped graphene was reported by Liu et al.435 The material was utilized as advanced anodes for lithium ion batteries. The Cl and N codoped system presented a specific capacitance of 1200 mAh/g at 0.1 A/g.
Among the theoretical investigations I can highlight my study about graphene with two 3p elements,436 which revealed unexpected properties because the addition of a second dopant decreased the band gaps with respect to the monodoped systems. Ullah and co-workers437−439 studied BeB, BeN,440 and BeO codoped graphene, which exhibited outstanding properties for alkali adsorption thanks to the presence of Be which makes the graphene sheet electron deficient. I studied codoped graphene with one 4p element and one 2p element, which exhibited remarkable properties when a perfect sheet was place above the codoped one because of the formation of interlayer bonds, in particular when Ga and Ge dopants were present.441 In the same line, Safaei Ardakani et al.442 studied theoretically SeX codoped graphene (X = Ga, P, and S), which exhibited outstanding electronic properties. N and Cl dopants were successfully incorporated into the graphene framework as revealed by the different characterization techniques employed.
Finally, again with the help of nitrogen, Se and N codoped graphene was prepared. Chen et al.443 synthesized codoped aerogels that showed a synergistically enhanced capacitive performance. The specific capacity was 302.9 F/g at 1 A/g. In my theoretical study about Se and N codoped graphene,441 I found that this system has the larger gap among the 4p/2p codoped graphenes: 0.83 eV (spin up) and 0.84 eV (spin down) at the M06-L/6-31G* level for a 4 atom % level of doping. Excellent activity for iodine reduction reaction was calculated by Zhong et al.444 for Se and N codoped graphene.
4.6. Transition Metal and Nitrogen Codoped Graphene
Along this review I have highlighted the key role played by nitrogen to synthesize codoped graphene systems. Although in section 2 I mentioned that Mn, Fe, Co, Ni, Ir, and Au metal dopants have been embedded in the graphene framework,80−86 nitrogen has been intensively used to stabilize metals in the graphene sheet.445−471 Among these systems Fe and N codoped graphene is one of the most studied ones,445−453 probably because they can be used in single-atom catalysis. In 2015, Dong et al.445 demonstrated that the latter system is a very effective catalyst for the ORR. These authors exfoliated graphite using cyclopentadienyl iron, and then upon pyrolysis and ammonia activation, the material was converted into Fe and N codoped graphene. The new catalyst exhibited excellent methanol tolerance, superior to that of commercial Pt/C. Other studies by Zitolo et al.,446 Niu et al.,447 and Jiang et al.448 also supported the strong catalytic power of Fe and N codoped graphene. In particular the study by Zitolo et al.446 revealed the existence of porphyrin-like FeN4C12 moieties. Sibul el al.449 found that Fe and N codoped graphene is a superior catalyst for anion exchange membranes rather than proton exchange membranes for fuel cell applications. A maximum power density of 243 mW/cm2 was obtained. Continuing with the use of Fe and N codoped graphene in catalysis, Zhang et al.450 employed density functional theory to show that by anchoring two Fe atoms with four nitrogen dopants it is possible to obtain an excellent catalyst for the oxygen reduction reaction. In a different area, Gao et al.451 reported a fluorometric and colorimetric dual-mode sensor based on nitrogen and iron codoped graphene quantum dots for detection of Fe3+ ions in biological fluids and cellular imaging. Finally, there is evidence452,453 indicating the usefulness of Fe and N codoped graphene in the development of lithium–sulfur batteries. Zhang et al. found that the codoped graphene can be used as an anchor material for sulfur in Li–S batteries. Li2S and Li2S2 presented very low decomposition energies on its surface.
Cu and N codoped graphene has been produced by Ni et al.454 by the thermal conversion of Cu(II) 2,2′-bipyridine in the confined space of lamellar montmorillonites. The product presented excellent results in terms of ORR catalytic activity and methanol tolerance in alkaline media. Mn and N codoped graphene has been the subject of several investigations.455−460 Zhu et al.455 employed density functional theory to show that this doped graphene is an excellent catalyst for the ORR, while Luo et al.456 employed first principles calculations to postulate that it can be a low-cost catalyst for CO oxidation at room temperature. Finally, Lee et al.457 prepared Co and N codoped graphene quantum dots used as bimodal resonance and fluorescence imaging nanoprobes.
In 2015, Li et al.458 performed a landmark theoretical investigation which proved that Co and N codoped graphene is a superior catalyst for the ORR and OER. It had a high selectivity for the four-electron-reduction pathway. These hypotheses were confirmed by experimental investigations.459−461 Han et al.459 ultrasonicated g-C3N4, glucose, and Co(CH3COOH)2·4H2O, and after several processes Co and N codoped carbon sheets were obtained. As usual for codoped carbons, the material had excellent ORR catalytic activity. The overpotential between ORR and OER reactions was 0.80 V at 10 mA/cm2. A similar behavior was observed by Liu et al.,460 but in this case the Co and N codoped graphene was synthesized using a simple Mg(OH)2 nanocasting method. In this case, the OER properties were similar to those corresponding to IrO2. Finally, Du et al.461 studied the use of Co and N codoped graphene as a single-atom catalyst for high sulfur content lithium–sulfur batteries, a result also supported by the work of Zhang et al.462 It is important to note that the latter work studied not only Co and N codoped graphene as an anchor material in Li–S batteries but also the V, Cr, Mn, Fe, Co, Ni, and Cu counterparts. The Co–N–C coordination center served as a bifunctional electrocatalyst that facilitated the formation of Li2S in discharge and its decomposition in the charge process. Finally, Ir and N codoped graphene which mimicked Ir porphyrins was synthesized by Xiao et al.,463 and it exhibited ORR catalytic activity significantly higher than that of Ir nanoparticles. These properties were attributed to the moderate adsorption energies of the intermediates. The idea of embedding metal atoms on N-doped graphene was extended to include more and different metals. Dual-metal and N-doped graphene has been predicted by theoretical calculations to have outstanding HER activity464 and catalytic effects for the CO2 reduction reaction.462
Other combinations of metals were used to codope graphene include; for example, Fe and S codoped graphene quantum dots were synthesized by Kharangarh et al.466 using a facile one-pot hydrothermal method. The material had excellent electrochemical properties and improved electrical conductivity. The specific capacitance was 476.2 F/g, about 3.3 times higher than that corresponding to the undoped material. Gu et al.467 reported the preparation of Ni and Al codoped graphene by the reduction of graphene oxide. The product had an impressive hydrogen storage uptake of 5.7 wt %, at 473 K.
4.7. Triple-Atom and Higher Codoped Graphene
The obvious question that some scientists asked was that if two are good why not three or more dopants? I studied XBN tridoped graphene where X = Al, Si, P, or S.468 As expected, the XNB motif was preferred because nitrogen stabilizes most dopants. The exception was sulfur, which preferred an SBN motif. In line with my previous findings for two dopants, the theoretical calculations indicated that in general the dopants considered preferred to be bonded instead of separated. The tridoped sheets presented interesting electronic properties and high reactivity. For AlNB, PNB, and SNB the carbon atoms are more reactive than in their AlN, PN, and SN codoped counterparts. However, for SiNB the reactivity is lower than that of SiN dual-doped graphene. As a consequence I recommended that, in order to increase reactivity, Al, P, and S should be combined with BN motifs. There is experimental evidence available indicating that the use of three dopants is beneficial. Razmjooei et al.469 prepared P, S, and N triple-doped graphene finding that its ORR activity is 2 times higher than that of S and N codoped graphene and 5 times higher than that of single P-doped graphene. Three dopants were also used by Wang et al.470 to capture the harmful bisphenol A, but in this case the dopants utilized were P, S, and N. The same combination of dopants was selected by Wang et al.471 to design a electrocatalyst of the ORR in alkaline medium. The material presented superior properties which indicated that it is a promising cathode catalyst for alkaline fuel cells. The good performance of P, S, and N triple-doped graphene for the ORR was also confirmed by the investigations of Dou et al.472 Last year, Wang et al.473 also prepared P, S, and N codoped graphene and showed that it is an excellent metal-free bifunctional catalyst for superior electrocatalytic oxygen reaction in rechargeable Zn–air batteries. The dopants were introduced by an interesting modified ball-milling process. Zheng et al.474 also incorporated P, S, and N codoped graphene as cathodes for Zn–air batteries, but in this case the triple-doped graphene was derived from onium salts. Finally, Xu et al.475 reported that P, S, and N tridoped graphene quantum dots are a very interesting ion fluorescence probe. The material was prepared from inexpensive coal, and fluorescence was quenched by Pb2+.
Some reports are focused on N, F, and S tridoped graphene476−479 and N, P, and S tridoped graphene.480 However, as I mentioned in the preceding sections, it is highly questionable whether fluorine is a dopant or not. Nevertheless, these materials were useful in catalysis,476,477,480 solar cells,478 and photoluminescence.479
B, N, and P tridoped graphene has been synthesized at least in two reports that observed excellent catalytic properties for the ORR.481,482 Lin et al.481 synthesized the material using boron phosphate and a B/P source and ammonia as the N-dopant agent. First graphene oxide was combined with boron phosphate to introduce the B and P dopants. After the B and P codoped graphene aerogel was obtained, it was activated with NH3 atmosphere to obtain the final tridoped graphene. P, N, and O tridoped graphene was reported by Zhao et al.483 and was proposed as a supercapacitor electrode and a metal-free catalyst for the oxygen reduction reaction. The specific capacitance was 426 F/g. Again the question of whether oxygen is a dopant is open because it is hard to evaluate if it is replacing a carbon atom or it is a functional group.
The high stabilization of metal dopants induced by graphene elicited several investigations of tridoped graphene containing two different metals and nitrogen. Hu et al.484 performed theoretical calculations which revealed that Fe, Co, and N tridoped graphene can be a superior catalyst of the ORR and OER. An extremely low overpotential of 0.22 V for both reactions was obtained. He and Santiago485 combined a variety of metal dimers on N-doped graphene, finding ultrahigh efficiency for the nitrogen reduction reaction. The landmark experimental and theoretical investigation by Zhou et al.486 proved that FeNi-N6 sites, where each metal is coordinated to four nitrogen atoms, dominate the catalytic activity of noble metal free catalysts. Excellent methane activation was indicated by the theoretical calculations performed by Wu et al.487 for tridoped graphene with two 3d metals and nitrogen.
Fe, S, and N tridoped graphene was obtained by Qiao et al.488 and Feng et al.489 In both works, it was fond that this tridoped graphene is an excellent catalyst of the ORR. Feng et al.489 obtained this material by using melamine and 2-aminotriazole. The addition of melamine increased the number of nitrogen atoms with pyridinic structure, and as a consequence the amount of FeNx was augmented.
Finally, I mention the study by Molina-García el al.490 published in 2018 that achieved the inclusion of four dopants in the graphene sheet: boron, nitrogen, phosphorus, and sulfur. The level of doping was determined via XPS as 6.4, 6.1, 2.6, and 0.5% for B, N, P, and S, respectively. The presence of P–N, P–C, B–C, and S–C bonds was confirmed among many other types. There was improved performance of the quaternary-doped graphene as indicated by the effective number of electron transferred: 3.2.
5. Conclusions and future prospects
In this review, I have presented a thorough analysis of the multiple atom doped graphene systems that have been synthesized and studied theoretically. I first raised the question of when a foreign atom can be considered a dopant. Although I have discussed works that claim to have doped graphene with fluorine, I believe that this atom cannot be considered a dopant but is a functional group. The case of oxygen is more complicated because it may possibly replace a carbon atom in graphene’s framework. However, if oxygen is considered a dopant in all the chemical forms that it can adopt, most of the single-atom graphene systems that have been prepared from graphene oxide are likely to be considered as codoped graphene systems. The amount of oxygen present may be similar to that achieved for the introduced dopant. Also, the effect of this residual oxygen on the properties studied should be clarified. Will it be the same if oxygen is not present?
Regarding the codoped graphene systems synthesized, the list includes (but is not limited to) B/N, N/O, N/F, S/N, P/N, P/B, P/O, S/P, S/B, Si/N, Cl/N, Se/N, TM/N (where TM is a metal), Fe/S, and Al/Ni. By far, the most studied system is S and N codoped graphene, followed by P/N, B/N, and TM/N codoped graphene. From this list of codoped systems, it is crystal clear that nitrogen dominates the list not only in the number of systems but also in the list of articles published because it is present in the four most studied codoped graphene systems. Therefore, the question emerges: are we codoping graphene or doping N-doped graphene? The strong electron-withdrawing properties of nitrogen and its ability to form multiple bonds are crucial for introducing other heteroatoms. Also, nitrogen can be present in various forms—pyridinic, graphitic, and pyrrolic—that can adapt to the needs of the dopant. For example, in B/N doped graphene, it may prefer to be in graphitic form to pair boron. However, for sulfur it can adopt a pyridinic structure, allowing sulfur to be present in thiophenic form CSC (see Figure 4). In the case of transition metals, they can be trapped in an N4 environment, as in porphyrins, anchoring the metal to graphene’s surface and avoiding the undesired metal clustering. Therefore, one of the field’s critical challenges is synthesizing new codoped graphene systems that do not include nitrogen. Finally, the question of what the effect is of the residual oxygen in the codoped graphene systems prepared remains open.
Related to the latter point, it is impossible not to mention the article by Martin Pumera’s research group11 asking if any “crap” that we put in graphene will enhance its catalytic activity. I believe this challenging paper has not received enough attention, and more studies are needed to give a final answer. In my opinion, one of the significant problems in the field is that the large number of methods available to synthesize codoped graphene makes it almost impossible to have a clear structure–property relationship. It is crucial to devise more promising approaches to synthesize codoped graphene systems with a specific disposition of the dopants. This may be attained, for example, by using adequate substrates as I proposed for siligraphene,88 or with the aid of specific molecular precursors as in the work by Nguyen et al.491 that reported the bottom-up synthesis of sulfur-doped graphene nanoribbons.
In this review, I have commented on how the multielemental doped graphene has been utilized in catalysis to develop new energy storage systems, sensing, protection against microwave radiation, piezoelectronics, and solar cells. Even though most of the works report outstanding properties, to the best of my knowledge, there is no widespread use of these nanomaterials in the industry. Maybe it is a bit soon because graphene was synthesized only 18 years ago and the time that doping has been investigated is even shorter. However, to achieve more maturity, this field needs an improved relationship with real-life applications so that society can realize how important graphene is. To that end, in my opinion, theory can be coupled with experiment to improve the synthesis protocols improving the structure–property relationship.
I expect that this review will contribute to introducing more researchers to this fascinating field and enlarge the list of codoped graphene systems that have been synthesized.
Acknowledgments
The author thanks PEDECIBA-Quimica, CSCI, and ANII for financial support.
The author declares no competing financial interest.
References
- Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Grigorieva; Dubonos S. V.; Firsov A. A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Katsnelson M. I.; Grigorieva I.; Dubonos S. V.; Firsov A. A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. 10.1038/nature04233. [DOI] [PubMed] [Google Scholar]
- Georgakilas V.; Otyepka M.; Bourlinos A. B.; Chandra V.; Kim N.; Kemp K. C.; Hobza P.; Zboril R.; Kim K. S. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112 (11), 6156–6214. 10.1021/cr3000412. [DOI] [PubMed] [Google Scholar]
- Denis P. A.Covalently Functionalized Graphene. Carbon Nanomaterials Sourcebook; CRC Press: 2016; pp 105–122. [Google Scholar]
- Wang X.; Sun G.; Routh P.; Kim D. H.; Huang W.; Chen P. Heteroatom-doped graphene materials: syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. 10.1039/C4CS00141A. [DOI] [PubMed] [Google Scholar]
- Terrones H.; Lv R.; Terrones M.; Dresselhaus M. S. The role of defects and doping in 2D graphene sheets and 1D nanoribbons. Rep. Prog. Phys. 2012, 75, 062501 10.1088/0034-4885/75/6/062501. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yu L.; Zhu W.; Zhou X.; Chen Y.; Peng W. Promotion of the performance of nitrogen-doped graphene by secondary heteroatoms doping in energy transformation and storage. Ionics 2019, 25, 3499–3522. 10.1007/s11581-019-03067-5. [DOI] [Google Scholar]
- Lee S. J.; Theerthagiri J.; Nithyadharseni P.; Arunachalam P.; Balaji D.; Madan Kumar A.; Madhavan J.; Mittal V.; Choi M. Y. Heteroatom-doped graphene-based materials for sustainable energy applications: A review. Renewable Sustainable Energy Rev. 2021, 143, 110849. 10.1016/j.rser.2021.110849. [DOI] [Google Scholar]
- Duan J.; Chen S.; Jaroniec M.; Qiao S. Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5 (9), 5207–5234. 10.1021/acscatal.5b00991. [DOI] [Google Scholar]
- Ullah S.; Shi Q.; Zhou J.; Yang X.; Ta H. Q.; Hasan M.; Ahmad N. M.; Fu L.; Bachmatiuk A.; Rummeli M. H. Advances and trends in chemically doped graphene. Adv. Mater. Interfaces 2020, 7, 2000999. 10.1002/admi.202000999. [DOI] [Google Scholar]
- Wang L.; Sofer Z.; Pumera M. Will any crap we put into graphene increase its electrocatalytic effect?. ACS Nano 2020, 14 (1), 21–25. 10.1021/acsnano.9b00184. [DOI] [PubMed] [Google Scholar]
- Kretschmer S.; Ghaderzadeh S.; Facsko S.; Krasheninnikov A. V. Threshold Ion Energies for Creating Defects in 2D Materials from First-Principles Calculations: Chemical Interactions Are Important. J. Phys. Chem. Lett. 2022, 13 (2), 514–519. 10.1021/acs.jpclett.1c03995. [DOI] [PubMed] [Google Scholar]
- Denis P. A. Lithium adsorption on heteroatom mono and dual doped graphene. Chem. Phys. Lett. 2017, 672, 70–79. 10.1016/j.cplett.2017.01.036. [DOI] [Google Scholar]
- Vineesh T. V.; Nazrulla M. A.; Krishnamoorthy S.; Narayanan T. N.; Alwarappan S. Synergistic effects of dopants on the spin density of catalytic active centres of N-doped fluorinated graphene for oxygen reduction reaction. Appl. Mater. Today 2015, 1, 74–79. 10.1016/j.apmt.2015.09.002. [DOI] [Google Scholar]
- Jiang S.; Sun Y.; Dai H.; Hu J.; Ni P.; Wang Y.; Li Z.; Li Z. Nitrogen and fluorine dual-doped mesoporous graphene: a high-performance metal-free ORR electrocatalyst with a super-low HO2–yield. Nanoscale 2015, 7, 10584–10589. 10.1039/C5NR01793A. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Feng Q.; Xu Q.; Li M.; Tang N.; Du Y. Synthesis and photoluminescence of F and N co-doped reduced graphene oxide. Carbon 2013, 61, 436–440. 10.1016/j.carbon.2013.05.027. [DOI] [Google Scholar]
- Yue X.; Huang S.; Cai J.; Jin Y.; Shen P. K. Heteroatoms dual doped porous graphene nanosheets as efficient bifunctional metal-free electrocatalysts for overall water-splitting. J. Mater. Chem. A 2017, 5, 7784–7790. 10.1039/C7TA01957B. [DOI] [Google Scholar]
- Yue X.; Huang S.; Jin Y.; Shen P. K. Nitrogen and fluorine dual-doped porous graphene-nanosheets as efficient metal-free electrocatalysts for hydrogen-evolution in acidic media. Catal. Sci. Technol. 2017, 7, 2228–2235. 10.1039/C7CY00384F. [DOI] [Google Scholar]
- Huang S.; Li Y.; Feng Y.; An H.; Long P.; Qin C.; Feng W. Nitrogen and fluorine co-doped graphene as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23095–23105. 10.1039/C5TA06012E. [DOI] [Google Scholar]
- An H.; Li Y.; Gao Y.; Cao C.; Han J.; Feng Y.; Feng W. Free-standing fluorine and nitrogen co-doped graphene paper as a high-performance electrode for flexible sodium-ion batteries. Carbon 2017, 116, 338–346. 10.1016/j.carbon.2017.01.101. [DOI] [Google Scholar]
- Wang C.; Chen D.; Yang Y.; Tang S.; Li X.; Xie F.; Wang G.; Guo Q. Synthesis of multi-color fluorine and nitrogen co-doped graphene quantum dots for use in tetracycline detection, colorful solid fluorescent ink, and film. J. Colloid Interface Sci. 2021, 602, 689–698. 10.1016/j.jcis.2021.06.062. [DOI] [PubMed] [Google Scholar]
- Wu A.; Wei G.; Yang F.; Xia G.; Yan X.; Shen S.; Zhu F.; Ke C.; Zhang J. Nitrogen and iodine dual-doped 3D porous graphene as a bi-functional cathode catalyst for Li-O2 batteries. Electrochim. Acta 2019, 318, 354–361. 10.1016/j.electacta.2019.05.099. [DOI] [Google Scholar]
- Zhan Y.; Huang J.; Lin Z.; Yu X.; Zeng D.; Zhang X.; Xie F.; Zhang W.; Chen J.; Meng H. Iodine/nitrogen co-doped graphene as metal free catalyst for oxygen reduction reaction. Carbon 2015, 95, 930–939. 10.1016/j.carbon.2015.09.024. [DOI] [Google Scholar]
- Park O.-K.; Kim H. J.; Hwang J. Y.; Lee D. S.; Koo J.; Lee H.; Yu J.-S.; Ku B.-C.; Lee J. K. Synthesis and mechanistic study of in situ halogen/nitrogen dual-doping in graphene tailored by stepwise pyrolysis of ionic liquids. Nanotechnology 2015, 26, 115601. 10.1088/0957-4484/26/11/115601. [DOI] [PubMed] [Google Scholar]
- Wang L.; Sofer Z.; Zboril R.; Cepe K.; Pumera M. Phosphorus and Halogen Co-Doped Graphene Materials and their Electrochemistry. Chem.—Eur. J. 2016, 22, 15444. 10.1002/chem.201602616. [DOI] [PubMed] [Google Scholar]
- Panchakarla L. S.; Subrahmanyam K. S.; Saha S. K.; Govindaraj A.; Krishnamurthy H. R.; Waghmare U. V.; Rao C. N. R. Synthesis, Structure, and Properties of Boron- and Nitrogen-Doped Graphene. Adv. Mater. 2009, 21, 4726–4730. 10.1002/adma.200901285. [DOI] [Google Scholar]
- Wang L.; Sofer Z.; Simek P.; Tomandl I.; Pumera M. Boron-Doped Graphene: Scalable and Tunable p-Type Carrier Concentration Doping. J. Phys. Chem. C 2013, 117, 23251–23257. 10.1021/jp405169j. [DOI] [Google Scholar]
- Laref A.; Ahmed A.; Binomran S.; Luo S. J. First-principle analysis of the electronic and optical properties of boron and nitrogen doped carbon mono-layer graphenes. Carbon 2015, 81, 179–192. 10.1016/j.carbon.2014.09.047. [DOI] [Google Scholar]
- Poh H. L.; Šimek P.; Sofer Z.; Tomandl I.; Pumera M. Boron and nitrogen doping of graphene via thermal exfoliation of graphite oxide in a BF3 or NH3 atmosphere: contrasting properties. J. Mater. Chem. A 2013, 1, 13146–13153. 10.1039/c3ta12460f. [DOI] [Google Scholar]
- Wang L.; Sofer Z.; Luxa J.; Pumera M. Nitrogen doped graphene: influence of precursors and conditions of the synthesis. J. Mater. Chem. C 2014, 2, 2887–2893. 10.1039/C3TC32359E. [DOI] [Google Scholar]
- Megawati M.; Chua C. K.; Sofer Z.; Klímová K.; Pumera M. Nitrogen-doped graphene: effect of graphite oxide precursors and nitrogen content on the electrochemical sensing properties. Phys. Chem. Chem. Phys. 2017, 19, 15914–15923. 10.1039/C7CP00520B. [DOI] [PubMed] [Google Scholar]
- Lv R.; Li Q.; Botello-Mendez A. R.; Hayashi T.; Wang B.; Berkdemir A.; Hao Q.; Elias A. L.; Cruz-Silva E.; Gutierrez H. R.; Kim Y. A.; Muramatsu H.; Zhu J.; Endo M.; Terrones H.; Charlier J.-C.; Pan M.; Terrones M. Nitrogen-doped graphene: beyond single substitution and enhanced molecular sensing. Sci. Rep. 2012, 2, 586. 10.1038/srep00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.; Li J.; Yang M.; Fang Z.; Jian J.; Yu D.; Chen X.; Dai L. Ultrathin black phosphorus-on-nitrogen doped graphene for efficient overall water splitting: dual modulation roles of directional interfacial charge transfer. J. Am. Chem. Soc. 2019, 141 (12), 4972–4979. 10.1021/jacs.9b00154. [DOI] [PubMed] [Google Scholar]
- Ullah S.; Denis P. A.; Sato F. Coupled cluster investigation of the interaction of beryllium, magnesium, and calcium with pyridine: Implications for the adsorption on nitrogen-doped graphene. Comput.Theor. Chem. 2019, 1150, 57–62. 10.1016/j.comptc.2019.01.015. [DOI] [Google Scholar]
- Vesel A.; Zaplotnik R.; Primc G.; Mozetič M. A Review of Strategies for the Synthesis of N-Doped Graphene-Like Materials. Nanomaterials 2020, 10, 2286. 10.3390/nano10112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah S.; Hussain A.; Syed W.; Saqlain M. A.; Ahmad I.; Leenaerts O.; Karim A. Band-gap tuning of graphene by Be doping and Be, B co-doping: a DFT study. RSC Adv. 2015, 5, 55762–55773. 10.1039/C5RA08061D. [DOI] [Google Scholar]
- Ullah S.; Denis P. A.; Sato F. Beryllium doped graphene as an efficient anode material for lithium-ion batteries with significantly huge capacity: A DFT study. Appl. Mater. Today 2017, 9, 333–340. 10.1016/j.apmt.2017.08.013. [DOI] [Google Scholar]
- Ao Z. M.; Yang J.; Li S.; Jiang Q. Enhancement of CO detection in Al doped graphene. Chem. Phys. Lett. 2008, 461, 276–279. 10.1016/j.cplett.2008.07.039. [DOI] [Google Scholar]
- Ao Z. M.; Li S.; Jiang Q. Thermal stability of interaction between the CO molecules and the Al doped graphene. Phys. Chem. Chem. Phys. 2009, 11, 1683–1687. 10.1039/b812188e. [DOI] [PubMed] [Google Scholar]
- Fukushima A.; Sawairi A.; Doi K.; Senami M.; Chen L.; Cheng H.; Tachibana A. Role of an Aluminum Atom on Graphene for Hydrogen Adsorption. J. Phys. Soc. Jpn. 2011, 80, 074705 10.1143/JPSJ.80.074705. [DOI] [Google Scholar]
- Tayyab M.; Hussain A.; Adil W.; Nabi S.; Asif Q. Band-gap engineering of graphene by Al doping and adsorption of Be and Br on impurity: A computational study. Comput. Cond. Matter 2020, 23, e00463 10.1016/j.cocom.2020.e00463. [DOI] [Google Scholar]
- Denis P. A. Band gap opening of monolayer and bilayer graphene doped with aluminium, silicon, phosphorus, and sulfur. Chem. Phys. Lett. 2010, 492, 251–257. 10.1016/j.cplett.2010.04.038. [DOI] [Google Scholar]
- Denis P. A. When noncovalent interactions are stronger than covalent bonds: Bilayer graphene doped with second row atoms, aluminum, silicon, phosphorus and sulfur. Chem. Phys. Lett. 2011, 508, 95–101. 10.1016/j.cplett.2011.04.018. [DOI] [Google Scholar]
- Denis P. A. Tuning the electronic properties of doped bilayer graphene with small structural changes. Comput. Theor. Chem. 2011, 974, 21–25. 10.1016/j.comptc.2011.07.006. [DOI] [Google Scholar]
- Su C.; Tripathi M.; Yan Q. B.; Wang Z.; Zhang Z.; Hofer C.; Wang H.; Basile L.; Su G.; Dong M.; Meyer J. C.; Kotakoski J.; Kong J.; Idrobo J. C.; Susi T.; Li J. Engineering single-atom dynamics with electron irradiation. Sci. Adv. 2019, 5, eaav2252. 10.1126/sciadv.aav2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah S.; Liu Y.; Hasan M.; Zeng W.; Shi Q.; Yang X.; Fu L.; Ta H. Q.; Lian X.; Sun J.; Yang R.; Liu L.; Rümmeli M. H. Direct synthesis of large-area Al-doped graphene by chemical vapor deposition: Advancing the substitutionally doped graphene family. Nano. Res. 2022, 15, 1310–1318. 10.1007/s12274-021-3655-x. [DOI] [Google Scholar]
- Zagler G.; Stecher M.; Trentino A.; Kraft F.; Su C.; Postl A.; Längle M.; Pesenhofer C.; Mangler C.; Åhlgren E. H.; Markevich A.; Zettl A.; Kotakoski J.; Susi T.; Mustonen K. Beam-driven dynamics of aluminium dopants in graphene. 2D Materials 2022, 9, 035009 10.1088/2053-1583/ac6c30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis P. A.; Pereyra Huelmo C. Structural characterization and chemical reactivity of dual doped graphene. Carbon 2015, 87, 106–115. 10.1016/j.carbon.2015.01.049. [DOI] [Google Scholar]
- Denis P. A.; Pereyra Huelmo C.; Martins A. S. Band Gap Opening in Dual-Doped Monolayer Graphene. J. Phys. Chem. C 2016, 120, 7103–7112. 10.1021/acs.jpcc.5b11709. [DOI] [Google Scholar]
- Zhou W.; Kapetanakis M. D.; Prange M. P.; Pantelides S. T.; Pennycook S. J.; Idrobo J.-C. Direct Determination of the Chemical Bonding of Individual Impurities in Graphene. Phys. Rev. Lett. 2012, 109, 206803. 10.1103/PhysRevLett.109.206803. [DOI] [PubMed] [Google Scholar]
- Ramasse Q. M.; Seabourne C. R.; Kepaptsoglou D.-M.; Zan R.; Bangert U.; Scott A. J. Probing the Bonding and Electronic Structure of Single Atom Dopants in Graphene with Electron Energy Loss Spectroscopy. Nano Lett. 2013, 13, 4989–4995. 10.1021/nl304187e. [DOI] [PubMed] [Google Scholar]
- Majeti V. K.; Roy A.; Gupta K. K.; Dey S. Effect of silicon dopant on mechanical properties of monolayer graphene. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 872, 012188 10.1088/1757-899X/872/1/012188. [DOI] [Google Scholar]
- Denis P. A.; Pereyra Huelmo C.; Iribarne F. On the band gaps and effective masses of mono and dual doped monolayer graphene. Comput. Mater. Sci. 2017, 137, 20–29. 10.1016/j.commatsci.2017.05.006. [DOI] [Google Scholar]
- Rafique M.; Shuai Y.; Hussain N. First-principles study on silicon atom doped monolayer graphene. Phys. E: Low-Dimens. Syst. Nanostructures 2018, 95, 94–101. 10.1016/j.physe.2017.09.012. [DOI] [Google Scholar]
- Houmad M.; Zaari H.; Benyoussef A.; El Kenz A.; Ez-Zahraouy H. Optical conductivity enhancement and band gap opening with silicon doped graphene. Carbon 2015, 94, 1021–1027. 10.1016/j.carbon.2015.07.033. [DOI] [Google Scholar]
- Denis P. A. Heteroatom promoted cycloadditions for graphene. ChemistrySelect 2016, 1, 5497–5500. 10.1002/slct.201601366. [DOI] [Google Scholar]
- Denis P. A. Organic chemistry of graphene: the Diels–Alder reaction. Chem.—Eur. J. 2013, 19, 15719–15725. 10.1002/chem.201302622. [DOI] [PubMed] [Google Scholar]
- Denis P. A. Diels-Alder reactions onto fluorinated and hydrogenated graphene. Chem. Phys. Lett. 2017, 684, 79–85. 10.1016/j.cplett.2017.06.034. [DOI] [Google Scholar]
- Denis P. A.; Pereyra Huelmo C.; Iribarne F. Cycloaddition reactions on epitaxial graphene. New J. Chem. 2019, 43, 11251–11257. 10.1039/C9NJ02528F. [DOI] [Google Scholar]
- Some S.; Kim J.; Lee K.; Kulkarni A.; Yoon Y.; Lee S.; Kim T.; Lee H. Highly Air-Stable Phosphorus-Doped n-Type Graphene Field-Effect Transistors. Adv. Mater. 2012, 24, 5481–5486. 10.1002/adma.201202255. [DOI] [PubMed] [Google Scholar]
- Li R.; Wei Z.; Gou X.; Xu W. Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts. RSC Adv. 2013, 3, 9978–9984. 10.1039/c3ra41079j. [DOI] [Google Scholar]
- Zhang C.; Mahmood N.; Yin H.; Liu F.; Hou Y. Synthesis of Phosphorus-Doped Graphene and its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries. Adv. Mater. 2013, 25, 4932–4937. 10.1002/adma.201301870. [DOI] [PubMed] [Google Scholar]
- Niu F.; Tao L. M.; Deng Y. C.; Wang Q. H.; Song W.-G. Phosphorus doped graphene nanosheets for room temperature NH3 sensing. New J. Chem. 2014, 38, 2269–2272. 10.1039/c4nj00162a. [DOI] [Google Scholar]
- Susi T.; Hardcastle T. P.; Hofsäss H.; Mittelberger A.; Pennycook T. J.; Mangler C.; Drummond-Brydson R.; Scott A. J.; Meyer J. C.; Kotakoski J. Single-atom spectroscopy of phosphorus dopants implanted into graphene. 2D Mater. 2017, 4, 021013 10.1088/2053-1583/aa5e78. [DOI] [Google Scholar]
- Gonzalez Larrude D.; Garcia-Basabe Y.; Freire F. L. Jr.; Rocco M. L. M. Electronic structure and ultrafast charge transfer dynamics of phosphorous doped graphene layers on a copper substrate: a combined spectroscopic study. RSC Adv. 2015, 5, 74189–74197. 10.1039/C5RA12799H. [DOI] [Google Scholar]
- Denis P. A. Concentration dependence of the band gaps of phosphorus and sulfur doped graphene. Comput. Mater. Sci. 2013, 67, 203–206. 10.1016/j.commatsci.2012.08.041. [DOI] [Google Scholar]
- Dai J.; Yuan J. Modulating the electronic and magnetic structures of P-doped graphene by molecule doping. J. Phys.: Condens. Matter 2010, 22, 225501. 10.1088/0953-8984/22/22/225501. [DOI] [PubMed] [Google Scholar]
- Gueorguiev G. K.; Furlan A.; Czigany Z.; Stafstrom S.; Hultman L. Intercalation of P atoms in Fullerene-like CPx. Chem. Phys. Lett. 2011, 501, 400–403. 10.1016/j.cplett.2010.11.024. [DOI] [Google Scholar]
- Poh H. L.; Sofer Z.; Novacek M.; Pumera M. Concurrent Phosphorus Doping and Reduction of Graphene Oxide. Chem.—Eur. J. 2014, 20, 4284–4291. 10.1002/chem.201304217. [DOI] [PubMed] [Google Scholar]
- Denis P. A.; Faccio R.; Mombru A. W. Is It Possible to Dope Single-Walled Carbon Nanotubes and Graphene with Sulfur?. ChemPhysChem 2009, 10, 715–722. 10.1002/cphc.200800592. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Yao Z.; Li G.; Fang G.; Nie H.; Liu Z.; Zhou X.; Chen X.; Huang S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6 (1), 205–211. 10.1021/nn203393d. [DOI] [PubMed] [Google Scholar]
- Yang S.; Zhi L.; Tang K.; Feng X.; Maier J.; Müllen K. Efficient Synthesis of Heteroatom (N or S)-Doped Graphene Based on Ultrathin Graphene Oxide-Porous Silica Sheets for Oxygen Reduction Reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. 10.1002/adfm.201200186. [DOI] [Google Scholar]
- Gao H.; Liu Z.; Song L.; Guo W.; Gao W.; Ci L.; Rao A.; Quan W.; Vajtai R.; Ajayan P. M Synthesis of S-doped graphene by liquid precursor. Nanotechnology 2012, 23, 275605. 10.1088/0957-4484/23/27/275605. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Li J.; Wang Z.; Wang X.; Feng W.; Zheng W.; Cao W.; Hu P. Low-Temperature Growth of Large-Area Heteroatom-Doped Graphene Film. Chem. Mater. 2014, 26, 2460–2466. 10.1021/cm500086j. [DOI] [Google Scholar]
- Wang Z.; Li P.; Chen Y.; He J.; Zhang W.; Schmidt O. G.; Li Y. Pure thiophene–sulfur doped reduced graphene oxide: synthesis, structure, and electrical properties. Nanoscale 2014, 6, 7281–7287. 10.1039/c3nr05061k. [DOI] [PubMed] [Google Scholar]
- Poh H. L.; Šimek P.; Sofer Z.; Pumera M. Sulfur-Doped Graphene via Thermal Exfoliation of Graphite Oxide in H2S, SO2, or CS2 Gas. ACS Nano 2013, 7, 5262–5272. 10.1021/nn401296b. [DOI] [PubMed] [Google Scholar]
- Denis P. A. Chemical Reactivity and Band-Gap Opening of Graphene Doped with Gallium, Germanium, Arsenic, and Selenium Atoms. ChemPhysChem 2014, 15, 3994–4000. 10.1002/cphc.201402608. [DOI] [PubMed] [Google Scholar]
- Tripathi M.; Markevich A.; Böttger R.; Facsko S.; Besley E.; Kotakoski J.; Susi T. Implanting Germanium into Graphene. ACS Nano 2018, 12 (5), 4641–4647. 10.1021/acsnano.8b01191. [DOI] [PubMed] [Google Scholar]
- Meng X.; Yu C.; Song X.; Iocozzia J.; Hong J.; Rager M.; Jin H.; Wang S.; Huang L.; Qiu J.; Lin Z. Scrutinizing Defects and Defect Density of Selenium-Doped Graphene for High-Efficiency Triiodide Reduction in Dye-Sensitized Solar Cells. Angew. Chem., Int. Ed. 2018, 57, 4682. 10.1002/anie.201801337. [DOI] [PubMed] [Google Scholar]
- Toh R. J.; Poh H. L.; Sofer Z.; Pumera M. Transition Metal (Mn, Fe, Co, Ni)-Doped Graphene Hybrids for Electrocatalysis. Chem.—Asian J. 2013, 8, 1295–1300. 10.1002/asia.201300068. [DOI] [PubMed] [Google Scholar]
- Carnevali V.; Patera L. L.; Prandini G.; Jugovac M.; Modesti S.; Comelli G.; Peressi M.; Africh C. Doping of epitaxial graphene by direct incorporation of nickel adatoms. Nanoscale 2019, 11, 10358–10364. 10.1039/C9NR01072F. [DOI] [PubMed] [Google Scholar]
- Wen X.; Duan Z.; Bai L.; Guan J. Atomic scandium and nitrogen-codoped graphene for oxygen reduction reaction. J. Power Sources 2019, 431, 265–273. 10.1016/j.jpowsour.2019.126650. [DOI] [Google Scholar]
- Robertson A. W.; Montanari B.; He K.; Kim J.; Allen C. S.; Wu Y. A.; Olivier J.; Neethling J.; Harrison N.; Kirkland A. I.; Warner J. H. Dynamics of Single Fe Atoms in Graphene Vacancies. Nano Lett. 2013, 13, 1468–1475. 10.1021/nl304495v. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Deng Q.; Bachmatiuk A.; Sandeep G.; Popov A.; Eckert J.; Rümmeli M. H. Free-standing single-atom-thick iron membranes suspended in graphene pores. Science 2014, 343, 1228–1232. 10.1126/science.1245273. [DOI] [PubMed] [Google Scholar]
- Lin P.-C.; Villarreal R.; Achilli S.; Bana H.; Nair M. N.; Tejeda A.; Verguts K.; De Gendt S.; Auge M.; Hofsäss H.; De Feyter S.; Di Santo G.; Petaccia L.; Brems S.; Fratesi G.; Pereira L. M. C. Doping Graphene with Substitutional Mn. ACS Nano 2021, 15 (3), 5449–5458. 10.1021/acsnano.1c00139. [DOI] [PubMed] [Google Scholar]
- Trentino A.; Mizohata K.; Zagler G.; Längle M.; Mustonen K.; Susi T.; Kotakoski J.; Åhlgren E. H. Two-step implantation of gold into graphene. 2D Mater. 2022, 9, 025011 10.1088/2053-1583/ac4e9c. [DOI] [Google Scholar]
- Li W.; Amiinu I. S.; Zhang B.; Zhang C.; Zhang Z.; Zhu J.; Liu J.; Pu Z.; Kou Z.; Mu S. Distorted niobium-self-doped graphene in-situ grown from 2D niobium carbide for catalyzing oxygen reduction. Carbon 2018, 139, 1144–1151. 10.1016/j.carbon.2018.08.020. [DOI] [Google Scholar]
- Pereyra Huelmo C.; Denis P. A. Silicon Carbide Induced Doping of Graphene: A New Potential Synthetic Route for SiC3 Siligraphene. J. Phys. Chem. C 2019, 123, 30341–30350. 10.1021/acs.jpcc.9b07978. [DOI] [Google Scholar]
- Sofer Z.; Jankovsky O.; Simek P.; Klimova K.; Mackova A.; Pumera M. Uranium and Thorium-Doped Graphene for Efficient Oxygen and Hydrogen Peroxide Reduction. ACS Nano 2014, 8, 7106–7114. 10.1021/nn502026k. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Ge L.; Jaroniec M.; Qiao S. Z.; Jiao Y. Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem., Int. Ed. 2013, 125, 3192–3198. 10.1002/ange.201209548. [DOI] [PubMed] [Google Scholar]
- Jung S.-M.; Lee E. K.; Choi M.; Shin D.; Jeon I.-Y.; Seo J.-M.; Jeong H. Y.; Park N.; Oh J. H.; Baek J.-B. Direct Solvothermal Synthesis of B/N-Doped Graphene. Angew. Chem., Int. Ed. 2014, 53, 2398–2401. 10.1002/anie.201310260. [DOI] [PubMed] [Google Scholar]
- Rani P.; Jindal V. K. Stability and electronic properties of isomers of B/N co-doped graphene. Appl. Nanosci. 2014, 4, 989–996. 10.1007/s13204-013-0280-3. [DOI] [Google Scholar]
- Rani P.; Dubey G. S.; Jindal V. K. DFT study of optical properties of pure and doped graphene. Physica E: Low-Dimensional Systems and Nanostructures 2014, 62, 28–35. 10.1016/j.physe.2014.04.010. [DOI] [Google Scholar]
- Wang B. Y.; Wang H.; Chen L. Y.; Hsueh H. C.; Li X.; Guo J.; Luo Y.; Chiou J. W.; Wang W. H.; Wang P. H.; Chen K. H.; Chen Y. C.; Chen L. C.; Chen C. H.; Wang J.; Pong W. F. Nonlinear bandgap opening behavior of BN co-doped graphene. Carbon 2016, 107, 857–864. 10.1016/j.carbon.2016.06.091. [DOI] [Google Scholar]
- Nath P.; Sanyal D.; Jana D. Semi-metallic to semiconducting transition in graphene nanosheet with site specific co-doping of boron and nitrogen. Phys. E: Low-Dimens. Syst. Nanostructures 2014, 56, 64–68. 10.1016/j.physe.2013.07.009. [DOI] [Google Scholar]
- Nascimento R.; Martins J. R.; Batista R. J. C.; Chacham H. Band gaps of BN-doped graphene: fluctuations, trends, and bounds. J. Phys. Chem. C 2015, 119 (9), 5055–5061. 10.1021/jp5101347. [DOI] [Google Scholar]
- Omidvar A. Electronic structure tuning and band gap opening of nitrogen and boron doped holey graphene flake: the role of single/dual doping. Mater. Chem. Phys. 2017, 202, 258–265. 10.1016/j.matchemphys.2017.09.025. [DOI] [Google Scholar]
- Novodchuk I.; Kayaharman M.; Ibrahim K.; Al-Tuairqi S.; Irannejad M.; Abdel-Rahman E.; Sanderson J.; Bajcsy M.; Yavuz M. B/N co-doped graphene oxide gel with extremely-high mobility and ION/IOFF for large-area field effect transistors. Carbon 2020, 158, 624–630. 10.1016/j.carbon.2019.11.034. [DOI] [Google Scholar]
- Zhu J.; He C.; Li Y.; Kang S.; Shen P. K. One-step synthesis of boron and nitrogen-dual-self-doped graphene sheets as non-metal catalysts for oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 14700–14705. 10.1039/c3ta13318d. [DOI] [Google Scholar]
- Xu C.; Su Y.; Liu D.; He X. Three-dimensional N, B-doped graphene aerogel as a synergistically enhanced metal-free catalyst for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 25440–25448. 10.1039/C5CP04211A. [DOI] [PubMed] [Google Scholar]
- Chen W.; Xu L.; Tian Y.; Li H.; Wang K. Boron and nitrogen co-doped graphene aerogels: facile preparation, tunable doping contents and bifunctional oxygen electrocatalysis. Carbon 2018, 137, 458–466. 10.1016/j.carbon.2018.05.061. [DOI] [Google Scholar]
- Zhang M.; Tao H.; Liu Y.; Yan C.; Hong S.; Masa J.; Robertson A. W.; Liu S.; Qiu J.; Sun Z. Ultrasound-assisted nitrogen and boron codoping of graphene oxide for efficient oxygen reduction reaction. ACS Sustainable Chem. Eng. 2019, 7, 3434–3442. 10.1021/acssuschemeng.8b05654. [DOI] [Google Scholar]
- Yuan R.; Xu Y.; Wang Y.; You F.; Chen W.; Ding C.; Jiang D.; Wang K. One-pot hydrothermal preparation of B and N co-doped graphene aerogels loaded with cobalt oxides for the synergistic enhancement of oxygen reduction. J. Electroanal. Chem. 2020, 877, 114555. 10.1016/j.jelechem.2020.114555. [DOI] [Google Scholar]
- Zhu J.; He G.; Tian Z.; Liang L.; Shen P. K. Facile synthesis of boron and nitrogen-dual-doped graphene sheets anchored platinum nanoparticles for oxygen reduction reaction. Electrochim. Acta 2016, 194, 276–282. 10.1016/j.electacta.2016.01.222. [DOI] [Google Scholar]
- Mazánek V.; Matějková S.; Sedmidubský D.; Pumera M.; Sofer Z. One-Step Synthesis of B/N Co-doped Graphene as Highly Efficient Electrocatalyst for the Oxygen Reduction Reaction: Synergistic Effect of Impurities. Chem.—Eur. J. 2018, 24, 928. 10.1002/chem.201704515. [DOI] [PubMed] [Google Scholar]
- Tai J.; Hu J.; Chen Z.; Lu H. Two-step synthesis of boron and nitrogen co-doped graphene as a synergistically enhanced catalyst for the oxygen reduction reaction. RSC Adv. 2014, 4, 61437–61443. 10.1039/C4RA10162F. [DOI] [Google Scholar]
- Kattel S.; Atanassov P.; Kiefer B. Density functional theory study of the oxygen reduction reaction mechanism in a BN co-doped graphene electrocatalyst. J. Mater. Chem. A 2014, 2, 10273–10279. 10.1039/c4ta01460j. [DOI] [Google Scholar]
- Han J.; Zhang Y.; Niu F.; Chen T.; Liu J.; Xu Y. Low-cost and highly efficient metal-free electrocatalysts for oxygen reduction reaction: environment-friendly three-dimensional B, N co-doped graphene aerogels. Electrocatalysis 2019, 10, 56–62. 10.1007/s12678-018-0494-y. [DOI] [Google Scholar]
- Huang S.; Zhang L.; Zhu J.; Shen P. K.; Jiang S. P. Crumpled nitrogen-and boron-dual-self-doped graphene sheets as an extraordinary active anode material for lithium ion batteries. J. Mater. Chem. A 2016, 4, 14155–14162. 10.1039/C6TA05623G. [DOI] [Google Scholar]
- Li F.; Su Y.; Zhao J. Shuttle inhibition by chemical adsorption of lithium polysulfides in B and N co-doped graphene for Li–S batteries. Phys. Chem. Chem. Phys. 2016, 18, 25241–25248. 10.1039/C6CP04071C. [DOI] [PubMed] [Google Scholar]
- Xiao F.; Lin Z.; Zhang J.; Lei Y.; Meng Y.; Chen X.; Zhao S.; Hong B.; Wang J.; Li D.; Xu J. A novel approach to facile synthesis of boron and nitrogen co-doped graphene and its application in lithium oxygen batteries. Energy Storage Mater. 2021, 41, 61–68. 10.1016/j.ensm.2021.05.042. [DOI] [Google Scholar]
- Jiang H. R.; Zhao T. S.; Shi L.; Tan P.; An L. First-Principles Study of Nitrogen-, Boron-Doped Graphene and Co-Doped Graphene as the Potential Catalysts in Nonaqueous Li–O2 Batteries. J. Phys. Chem. C 2016, 120 (12), 6612–6618. 10.1021/acs.jpcc.6b00136. [DOI] [Google Scholar]
- Wang M.; Yang Y.; Yang Z.; Gu L.; Chen Q.; Yu Y. Sodium-ion batteries: improving the rate capability of 3D interconnected carbon nanofibers thin film by boron, nitrogen dual-doping. Adv. Sci. 2017, 4, 1600468. 10.1002/advs.201600468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.; Wang B.; Yan Y.; Rana H. H.; Lee J. Y.; Kim J. H.; Park H. S. Three-dimensionally macroporous nitrogen and boron co-doped graphene aerogels derived from polyaspartamide for supercapacitor electrodes. Mater. Today Commun. 2020, 25, 101495. 10.1016/j.mtcomm.2020.101495. [DOI] [Google Scholar]
- Wu Z. S.; Winter A.; Chen L.; Sun Y.; Turchanin A.; Feng X.; Müllen K. Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 2012, 24, 5130–5135. 10.1002/adma.201201948. [DOI] [PubMed] [Google Scholar]
- Tabassum H.; Mahmood A.; Wang Q.; Xia W.; Liang Z.; Qiu B.; Zhao R.; Zou R. Hierarchical cobalt hydroxide and B/N Co-doped graphene nanohybrids derived from metal-organic frameworks for high energy density asymmetric supercapacitors. Sci. Rep. 2017, 7, 43084. 10.1038/srep43084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.; Pei S.; He Z.; Shao H.; Wang J.; Wang K.; Wang Y.; Jin Y. High active PdSn binary alloyed catalysts supported on B and N codoped graphene for formic acid electro-oxidation. Catalysts 2020, 10, 751. 10.3390/catal10070751. [DOI] [Google Scholar]
- Sun Y.; Du C.; Han G.; Qu Y.; Du L.; Wang Y.; Chen G.; Gao Y.; Yin G. Boron, nitrogen co-doped graphene: a superior electrocatalyst support and enhancing mechanism for methanol electrooxidation. Electrochim. Acta 2016, 212, 313–321. 10.1016/j.electacta.2016.06.168. [DOI] [Google Scholar]
- Yang F.; Cao Y.; Chen Z.; He X.; Hou L.; Li Y. Large-scale preparation of B/N co-doped graphene-like carbon as an efficient metal-free catalyst for the reduction of nitroarenes. New J. Chem. 2018, 42, 2718–2725. 10.1039/C7NJ04187J. [DOI] [Google Scholar]
- Yu C.; Fang H.; Liu Z.; Hu H.; Meng X.; Qiu J. Chemically grafting graphene oxide to B, N co-doped graphene via ionic liquid and their superior performance for triiodide reduction. Nano Energy 2016, 25, 184–192. 10.1016/j.nanoen.2016.04.039. [DOI] [Google Scholar]
- Feng S.; Meng J.; Guo J.; Chen X.; Zhang G. B, N dual-doped Graphene/Au@ Pt Nanomaterials as Sensor for Determination of Aflatoxin B1. Int. J. Electrochem. Sci. 2020, 15, 7722–7732. 10.20964/2020.08.97. [DOI] [Google Scholar]
- Liu Z.; Mo Z.; Niu X.; Yang X.; Jiang Y.; Zhao P.; Liu N.; Guo R. Highly sensitive fluorescence sensor for mercury (II) based on boron- and nitrogen-co-doped graphene quantum dots. J. Colloid Interface Sci. 2020, 566, 357–368. 10.1016/j.jcis.2020.01.092. [DOI] [PubMed] [Google Scholar]
- Yang P.; Su J.; Guo R.; Yao F.; Yuan C. B, N-Co-doped graphene quantum dots as fluorescence sensor for detection of Hg2+ and F– ions. Anal. Methods 2019, 11, 1879–1883. 10.1039/C9AY00249A. [DOI] [Google Scholar]
- Yang G. H.; Zhou Y. H.; Wu J. J.; Cao J. T.; Li L. L.; Liu H. Y.; Zhu J. J. Microwave-assisted synthesis of nitrogen and boron co-doped graphene and its application for enhanced electrochemical detection of hydrogen peroxide. RSC Adv. 2013, 3, 22597–22604. 10.1039/c3ra44284e. [DOI] [Google Scholar]
- Varodi C.; Pogăcean F.; Coros M.; Magerusan L.; Stefan-van Staden R. I.; Pruneanu S. Hydrothermal Synthesis of Nitrogen, Boron Co-Doped Graphene with Enhanced Electro-Catalytic Activity for Cymoxanil Detection. Sensors 2021, 21, 6630. 10.3390/s21196630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esrafili M. D. Boron and nitrogen co-doped graphene nanosheets for NO and NO2 gas sensing. Phys. Lett. A 2019, 383, 1607–1604. 10.1016/j.physleta.2019.02.017. [DOI] [Google Scholar]
- Budak E.; Ünlü C. Boron regulated dual emission in B, N doped graphene quantum dots. Opt. Mater. 2021, 111, 110577. 10.1016/j.optmat.2020.110577. [DOI] [Google Scholar]
- Wang H.; Mu Q.; Wang K.; Revia R. A.; Yen C.; Gu X.; Tian B.; Liu J.; Zhang M. Nitrogen and boron dual-doped graphene quantum dots for near-infrared second window imaging and photothermal therapy. Appl. Mater. Today 2019, 14, 108–107. 10.1016/j.apmt.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngidi N. P. D.; Muchuweni E.; Nyamori V. O. Dual heteroatom-doped reduced graphene oxide and its application in dye-sensitized solar cells. Opt. Mater. 2021, 122, 111689. 10.1016/j.optmat.2021.111689. [DOI] [Google Scholar]
- Lin K. Y.; Nguyen M. T.; Waki K.; Jiang J. C. Boron and nitrogen co-doped graphene used as counter electrode for iodine reduction in dye-sensitized solar cells. J. Phys. Chem. C 2018, 122 (46), 26385–26392. 10.1021/acs.jpcc.8b06956. [DOI] [Google Scholar]
- Wang S.; Feng J.; Meng Q.; Cao B.; Tian G. Study on boron and nitrogen co-doped graphene xerogel for high-performance electrosorption application. J. Solid State Electrochem. 2019, 23, 2377–2390. 10.1007/s10008-019-04336-z. [DOI] [Google Scholar]
- Sun Z.; Yan Z.; Yue K.; Li A.; Qian L. Novel high-performance electromagnetic absorber based on Nitrogen/Boron co-doped reduced graphene oxide. Compos. B. Eng. 2020, 196, 108132. 10.1016/j.compositesb.2020.108132. [DOI] [Google Scholar]
- Umrao S.; Gupta T. K.; Kumar S.; Singh V. K.; Sultania M. K.; Jung J. H.; Oh I.-K.; Srivastava A. Microwave-assisted synthesis of boron and nitrogen co-doped reduced graphene oxide for the protection of electromagnetic radiation in Ku-band. ACS Appl. Mater. Interfaces 2015, 7 (35), 19831–19842. 10.1021/acsami.5b05890. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Yan F.; Li C.; Qi L.; Yuan H.; Liu Y.; Zhu C.; Chen Y. Nitrogen and boron co-doped carbon nanotubes embedded with nickel nanoparticles as highly efficient electromagnetic wave absorbing material. Chin. Phys. Lett. 2021, 38, 015201 10.1088/0256-307X/38/1/015201. [DOI] [Google Scholar]
- Chen S.; Duan J.; Jaroniec M.; Qiao S. Z. Nitrogen and Oxygen Dual-Doped Carbon Hydrogel Film as a Substrate-Free Electrode for Highly Efficient Oxygen Evolution Reaction. Adv. Mater. 2014, 26, 2925–2930. 10.1002/adma.201305608. [DOI] [PubMed] [Google Scholar]
- Qin T.; Wan Z.; Wang Z.; Wen Y.; Liu M.; Peng S.; et al. 3D flexible O/N Co-doped graphene foams for supercapacitor electrodes with high volumetric and areal capacitances. J. Power Sources 2016, 336, 455–464. 10.1016/j.jpowsour.2016.11.003. [DOI] [Google Scholar]
- Li Z.; Mi H.; Bai Z.; Ji C.; Sun L.; Gao S.; Qiu J. Sustainable biowaste strategy to fabricate dual-doped carbon frameworks with remarkable performance for flexible solid-state supercapacitors. J. Power Sources 2019, 418, 112–121. 10.1016/j.jpowsour.2019.02.034. [DOI] [Google Scholar]
- Li Z.; Li Y.; Wang L.; Cao L.; Liu X.; Chen Z.; Pan D.; Wu M. Assembling nitrogen and oxygen co-doped graphene quantum dots onto hierarchical carbon networks for all-solid-state flexible supercapacitors. Electrochim. Acta 2017, 235, 561–569. 10.1016/j.electacta.2017.03.147. [DOI] [Google Scholar]
- Li Z.; Cao L.; Qin P.; Liu X.; Chen Z.; Wang L.; Pan D.; Wu M. Nitrogen and oxygen co-doped graphene quantum dots with high capacitance performance for micro-supercapacitors. Carbon 2018, 139, 67–75. 10.1016/j.carbon.2018.06.042. [DOI] [Google Scholar]
- Mi K.; Chen S.; Xi B.; Kai S.; Jiang Y.; Feng J.; Qian Y.; Xiong S. Sole chemical confinement of polysulfides on nonporous nitrogen/oxygen dual-doped carbon at the kilogram scale for lithium–sulfur batteries. Adv. Funct. Mater. 2017, 27, 1604265. 10.1002/adfm.201604265. [DOI] [Google Scholar]
- Shi M.; Zhang S.; Jiang Y.; Jiang Z.; Zhang L.; Chang J.; Wei T.; Fan Z. Sandwiching sulfur into the dents between N, O Co-doped graphene layered blocks with strong physicochemical confinements for stable and high-rate Li–S batteries. Nano-Micro Lett. 2020, 12, 146. 10.1007/s40820-020-00477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.; Zhang Y.; Huang J.; Wang Y.; Li H.; Hwang B. J.; Zhao J. Nitrogen and oxygen dual-doped hollow carbon nanospheres derived from catechol/polyamine as sulfur hosts for advanced lithium sulfur batteries. Carbon 2017, 124, 23–33. 10.1016/j.carbon.2017.08.035. [DOI] [Google Scholar]
- Sun Y.; Zhu D.; Liang Z.; Zhao Y.; Tian W.; Ren X.; Wang J.; Li X.; Gao Y.; Wen W.; Huang Y.; Li X.; Tai R. Facile renewable synthesis of nitrogen/oxygen co-doped graphene-like carbon nanocages as general lithium-ion and potassium-ion batteries anode. Carbon 2020, 167, 685–695. 10.1016/j.carbon.2020.06.046. [DOI] [Google Scholar]
- Zhu Y.; Wang M.; Zhang Y.; Wang R.; Zhang Y.; Wang C. Nitrogen/oxygen dual-doped hierarchically porous carbon/graphene composite as high-performance anode for potassium storage. Electrochim. Acta 2021, 377, 138093. 10.1016/j.electacta.2021.138093. [DOI] [Google Scholar]
- Yang J.; Ju Z.; Jiang Y.; Xing Z.; Xi B.; Feng J.; Xiong S. Enhanced capacity and rate capability of nitrogen/oxygen dual-doped hard carbon in capacitive potassium-ion storage. Adv. Mater. 2018, 30, 1700104. 10.1002/adma.201700104. [DOI] [PubMed] [Google Scholar]
- Ruan J.; Zhao Y.; Luo S.; Yuan T.; Yang J.; Sun D.; Zheng S. Fast and stable potassium-ion storage achieved by in situ molecular self-assembling N/O dual-doped carbon network. Energy Storage Mater. 2019, 23, 46–54. 10.1016/j.ensm.2019.05.037. [DOI] [Google Scholar]
- Liang J.; Jiao Y.; Jaroniec M.; Qiao S. Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem., Int. Ed. 2012, 51, 11496–11500. 10.1002/anie.201206720. [DOI] [PubMed] [Google Scholar]
- Denis P. A.; Huelmo C. P.; Iribarne F. Theoretical characterization of sulfur and nitrogen dual-doped graphene. Comp. Theor Chem. 2014, 1049, 13–19. 10.1016/j.comptc.2014.08.023. [DOI] [Google Scholar]
- Paraknowitsch J. P.; Wienert B.; Zhang Y.; Thomas A. Intrinsically Sulfur- and Nitrogen-Co-doped Carbons from Thiazolium Salts. Chem.—Eur. J. 2012, 18, 15416–15423. 10.1002/chem.201202445. [DOI] [PubMed] [Google Scholar]
- Su Y.; Zhang Y.; Zhuang X.; Li S.; Wu D.; Zhang F.; Feng X. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon 2013, 62, 296–301. 10.1016/j.carbon.2013.05.067. [DOI] [Google Scholar]
- Denis P. A.; Iribarne F. The effect of the dopant nature on the reactivity, interlayer bonding and electronic properties of dual doped bilayer graphene. Phys. Chem. Chem. Phys. 2016, 18, 24693–24703. 10.1039/C6CP02481E. [DOI] [PubMed] [Google Scholar]
- Ullah S.; Denis P. A.; Sato F. Unusual Enhancement of the Adsorption Energies of Sodium and Potassium in Sulfur–Nitrogen and Silicon–Boron Codoped Graphene. ACS Omega 2018, 3, 15821–15828. 10.1021/acsomega.8b02500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.; Ning G.; Sun Y.; Pu Y.; Gao J. High capacity Li storage in sulfur and nitrogen dual-doped graphene networks. Carbon 2014, 79, 310–320. 10.1016/j.carbon.2014.07.072. [DOI] [Google Scholar]
- You J. M.; Ahmed M. S.; Han H. S.; Choe J.; Ustundag Z.; Jeon S. New approach of nitrogen and sulfur-doped graphene synthesis using dipyrrolemethane and their electrocatalytic activity for oxygen reduction in alkaline media. J. Power Sources 2015, 275, 73–79. 10.1016/j.jpowsour.2014.10.174. [DOI] [Google Scholar]
- Kicinski W.; Norek M.; Dziura A.; Polanski M. Copolycondensation of heterocyclic aldehydes: A general approach to sulfur and nitrogen dually-doped carbon gels. Microporous Mesoporous Mater. 2016, 225, 198–209. 10.1016/j.micromeso.2015.11.050. [DOI] [Google Scholar]
- Chen Q.; Liu H.; Zhu R.; Wang X.; Wang S.; Zhu J.; He H. Facile synthesis of nitrogen and sulfur co-doped graphene-like carbon materials using methyl blue/montmorillonite composites. Microporous Mesoporous Mater. 2016, 225, 137–143. 10.1016/j.micromeso.2015.12.026. [DOI] [Google Scholar]
- Feng B.; Xie J.; Dong C.; Zhang S.; Cao G.; Zhao X. From graphite oxide to nitrogen and sulfur co-doped few-layered graphene by a green reduction route via Chinese medicinal herbs. RSC Adv. 2014, 4, 17902–17907. 10.1039/c4ra01985g. [DOI] [Google Scholar]
- Wu M.; Liu Y.; Zhu Y.; Lin J.; Liu J.; Hu H.; Wang Y.; Zhao Q.; Lv R.; Qiu J. Supramolecular polymerization-assisted synthesis of nitrogen and sulfur dual-doped porous graphene networks from petroleum coke as efficient metal-free electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 11331–11339. 10.1039/C7TA03264A. [DOI] [Google Scholar]
- Zhang H.; Liu X.; He G.; Zhang X.; Bao S.; Hu W. Bioinspired synthesis of nitrogen/sulfur co-doped graphene as an efficient electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 279, 252–258. 10.1016/j.jpowsour.2015.01.016. [DOI] [Google Scholar]
- Carraro F.; Cattelan M.; Favaro M.; Calvillo L. Aerosol Synthesis of N and NS Doped and Crumpled Graphene Nanostructures. Nanomaterials 2018, 8, 406. 10.3390/nano8060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Zhang Y.; Zhang X.; Huang J.; Han J.; Zhang Z.; Han X.; Xu P.; Song B. S, N Dual-Doped Graphene-like Carbon Nanosheets as Efficient Oxygen Reduction Reaction Electrocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 398–405. 10.1021/acsami.6b12547. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Li P.; Yin X.; Yan Y.; Zhan K.; Yang J.; Zhao B. Cobalt sulfide supported on nitrogen and sulfur dual-doped reduced graphene oxide for highly active oxygen reduction reaction. RSC Adv. 2017, 7, 50246–50253. 10.1039/C7RA09231H. [DOI] [Google Scholar]
- Akhter T.; Islam M. M.; Faisal S. N.; Haque E.; Minett A. I.; Liu H. K.; Konstantinov K.; Dou S. X. Self-assembled N/S codoped flexible graphene paper for high performance energy storage and oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8 (3), 2078–2087. 10.1021/acsami.5b10545. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Liu Y.; Quan X.; Chen S.; Zhao H.; Yu H. Nitrogen and sulfur co-doped graphene/carbon nanotube as metal-free electrocatalyst for oxygen evolution reaction: the enhanced performance by sulfur doping. Electrochim. Acta 2016, 204, 169–175. 10.1016/j.electacta.2016.04.034. [DOI] [Google Scholar]
- Zhang X.; Wen X.; Pan C.; Xiang X.; Hao C.; Meng Q.; Tian Z. Q.; Shen P. K.; Jiang S. P. N species tuning strategy in N, S co-doped graphene nanosheets for electrocatalytic activity and selectivity of oxygen redox reactions. Chem. Eng. J. 2022, 431, 133216. 10.1016/j.cej.2021.133216. [DOI] [Google Scholar]
- Wu M.; Wang J.; Wu Z.; Xin H. L.; Wang D. Synergistic enhancement of nitrogen and sulfur co-doped graphene with carbon nanosphere insertion for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 7727–7731. 10.1039/C4TA06323F. [DOI] [Google Scholar]
- Higgins D. C.; Hoque M. A.; Hassan F.; Choi J. Y.; Kim B.; Chen Z. Oxygen reduction on graphene–carbon nanotube composites doped sequentially with nitrogen and sulfur. ACS Catal. 2014, 4 (8), 2734–2740. 10.1021/cs5003806. [DOI] [Google Scholar]
- Pan F.; Duan Y.; Zhang X.; Zhang J. A facile synthesis of nitrogen/sulfur co-doped graphene for the oxygen reduction reaction. ChemCatChem 2016, 8, 163–170. 10.1002/cctc.201500893. [DOI] [Google Scholar]
- Xu J.; Dong G.; Jin C.; Huang M.; Guan L. Sulfur and nitrogen co-doped, few-layered graphene oxide as a highly efficient electrocatalyst for the oxygen-reduction reaction. ChemSusChem 2013, 6, 493–499. 10.1002/cssc.201200564. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; Xia Z. Design principles for dual-element-doped carbon nanomaterials as efficient bifunctional catalysts for oxygen reduction and evolution reactions. ACS Catal. 2016, 6, 1553–1558. 10.1021/acscatal.5b02731. [DOI] [Google Scholar]
- Wu D.; Wang T.; Wang L.; Jia D. Hydrothermal synthesis of nitrogen, sulfur co-doped graphene and its high performance in supercapacitor and oxygen reduction reaction. Microporous Mesoporous Mater. 2019, 290, 109556. 10.1016/j.micromeso.2019.06.018. [DOI] [Google Scholar]
- Wu M.; Dou Z.; Chang J.; Cui L. Nitrogen and sulfur co-doped graphene aerogels as an efficient metal-free catalyst for oxygen reduction reaction in an alkaline solution. RSC Adv. 2016, 6, 22781–22790. 10.1039/C5RA22136F. [DOI] [Google Scholar]
- He G.; Qiao M.; Li W.; Lu Y.; Zhao T.; Zou R.; Li B.; Darr S. J.A.; Hu J.; Titirici M. M.; Parkin I. P. N-Co-doped graphene-nickel cobalt sulfide aerogel: improved energy storage and electrocatalytic performance. Adv. Sci. 2017, 4, 1600214. 10.1002/advs.201600214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi H.; Mehrpooya M. Synthesis of three-metal layered double hydroxide and dual doped graphene oxide composite as a novel electrocatalyst for oxygen reduction reaction. J. Alloys Compd. 2021, 875, 160047. 10.1016/j.jallcom.2021.160047. [DOI] [Google Scholar]
- Ma R.; Xia B. Y.; Zhou Y.; Li P.; Chen Y.; Liu Q.; Wang J. Ionic liquid-assisted synthesis of dual-doped graphene as efficient electrocatalysts for oxygen reduction. Carbon 2016, 102, 58–65. 10.1016/j.carbon.2016.02.034. [DOI] [Google Scholar]
- Zhang W.; Zhang Y.; Li Y.; Yang S.; Zhang L.-H.; Yu F. Enhanced oxygen reduction performance of nitrogen and sulfur Co-doped graphene oxide by immobilized ionic liquid. Chem. Eng. J. 2021, 236, 116544. 10.1016/j.ces.2021.116544. [DOI] [Google Scholar]
- Cui Z.; Wang S.; Zhang Y.; Cao M. A simple and green pathway toward nitrogen and sulfur dual doped hierarchically porous carbons from ionic liquids for oxygen reduction. J. Power Sources 2014, 259, 138–144. 10.1016/j.jpowsour.2014.02.084. [DOI] [Google Scholar]
- Moosapour Siahkalroudi Z.; Aghabarari B.; Vaezi M.; Rodríguez-Castellón E.; Martínez-Huerta M. V. Effect of secondary heteroatom (S, P) in N-doped reduced graphene oxide catalysts to oxygen reduction reaction. Mol. Catal. 2021, 502, 111372. 10.1016/j.mcat.2020.111372. [DOI] [Google Scholar]
- Tang Y.; Jing F.; Xu Z.; Zhang F.; Mai Y.; Wu D. Highly Crumpled Hybrids of Nitrogen/Sulfur Dual-Doped Graphene and Co9S8 Nanoplates as Efficient Bifunctional Oxygen Electrocatalysts. ACS Appl. Mater. Interfaces 2017, 9 (14), 12340–12347. 10.1021/acsami.6b15461. [DOI] [PubMed] [Google Scholar]
- Peng W.; Jin J.; Yang S.; Shen Z.; Wang H.; Zhang J.; Li G. Hollow Co9S8 spheres-derived polyhedrons uniformly anchored on N, S-doped graphene for efficient oxygen electrocatalysts. Compos. Commun. 2021, 23, 100587. 10.1016/j.coco.2020.100587. [DOI] [Google Scholar]
- Ma X. X.; Su Y.; He X. Q. Fe9S10-decorated N, S co-doped graphene as a new and efficient electrocatalyst for oxygen reduction and oxygen evolution reactions. Catal. Sci. Technol. 2017, 7, 1181–1192. 10.1039/C6CY02215D. [DOI] [Google Scholar]
- Zhang M.; Hong W.; Xue R.; Li L.; Huang G.; Xu X.; Gao J.; Yan J. Nitrogen/sulfur dual-doped reduced graphene oxide supported CuFeS2 as an efficient electrocatalyst for the oxygen reduction reaction. New J. Chem. 2018, 42, 2081–2088. 10.1039/C7NJ03204H. [DOI] [Google Scholar]
- Wang X.; Wang J.; Wang D.; Dou S.; Ma Z.; Wu J.; Tao L.; Shen A.; Ouyang C.; Liu Q.; Wang S. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. 10.1039/C4CC00440J. [DOI] [PubMed] [Google Scholar]
- Chae G. S.; Youn D. H.; Lee J. S. Nanostructured iron sulfide/N, S dual-doped carbon nanotube-graphene composites as efficient electrocatalysts for oxygen reduction reaction. Materials 2021, 14, 2146. 10.3390/ma14092146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibul R.; Kibena-Põldsepp E.; Mäeorg U.; Merisalu M.; Kikas A.; Kisand V.; Treshchalov A.; Sammelselg V.; Tammeveski K. Sulphur and nitrogen co-doped graphene-based electrocatalysts for oxygen reduction reaction in alkaline medium. Electrochem. Commun. 2019, 109, 106603. 10.1016/j.elecom.2019.106603. [DOI] [Google Scholar]
- Ye X.; Hu L.; Liu M.; Wang G.; Yu F. Improved oxygen reduction performance of a N, S co-doped graphene-like carbon prepared by a simple carbon bath method. New Carbon Mater. 2020, 35, 531–539. 10.1016/S1872-5805(20)60506-6. [DOI] [Google Scholar]
- Rivera-Gavidia L. M.; Luis-Sunga M.; Bousa M.; Vales V.; Kalbac M.; Arevalo M. C.; Pastor E.; Garcia G. S-and N-doped graphene-based catalysts for the oxygen evolution reaction. Electrochim. Acta 2020, 340, 135975. 10.1016/j.electacta.2020.135975. [DOI] [Google Scholar]
- Fan T.; Zhang G.; Jian L.; Murtaza I.; Meng H.; Liu Y.; Min Y. Facile synthesis of defect-rich nitrogen and sulfur Co-doped graphene quantum dots as metal-free electrocatalyst for the oxygen reduction reaction. J. Alloys Compd. 2019, 792, 844–850. 10.1016/j.jallcom.2019.04.097. [DOI] [Google Scholar]
- Mousavi S. A.; Mehrpooya M. Fabrication of copper centered metal organic framework and nitrogen, sulfur dual doped graphene oxide composite as a novel electrocatalyst for oxygen reduction. Energy 2021, 214, 119053. 10.1016/j.energy.2020.119053. [DOI] [Google Scholar]
- Chabu J. M.; Wang L.; Tang F. Y.; Zeng K.; Sheng J.; Walle M. D.; Deng L.; Liu Y. N. Synthesis of Three-Dimensional Nitrogen and Sulfur Dual-Doped Graphene Aerogels as an Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. ChemElectroChem. 2017, 4, 1885–1890. 10.1002/celc.201700002. [DOI] [Google Scholar]
- Li Y.; Yang J.; Huang J.; Zhou Y.; Xu K.; Zhao N.; Cheng X. Soft template-assisted method for synthesis of nitrogen and sulfur co-doped three-dimensional reduced graphene oxide as an efficient metal free catalyst for oxygen. Carbon 2017, 122, 237–246. 10.1016/j.carbon.2017.06.046. [DOI] [Google Scholar]
- Lei Z.; Feng W.; Feng C.; Zhou W.; Wei C.; Wang X. Nitrified coke wastewater sludge flocs: an attractive precursor for N, S dual-doped graphene-like carbon with ultrahigh capacitance and oxygen reduction performance. J. Mater. Chem. A 2017, 5, 2012–2020. 10.1039/C6TA09887H. [DOI] [Google Scholar]
- Zhao C.; Li J.; Chen Y.; Chen J. Nitrogen and sulfur dual-doped graphene as an efficient metal-free electrocatalyst for the oxygen reduction reaction in microbial fuel cells. New J. Chem. 2019, 43, 9389–9395. 10.1039/C9NJ01480B. [DOI] [Google Scholar]
- Bag S.; Mondal B.; Das A. K.; Raj C. R. Nitrogen and sulfur dual-doped reduced graphene oxide: synergistic effect of dopants towards oxygen reduction reaction. Electrochim. Acta 2015, 163, 16–23. 10.1016/j.electacta.2015.02.130. [DOI] [Google Scholar]
- Amiinu I. S.; Zhang J.; Kou Z.; Liu X.; Asare O. K.; Zhou H.; Cheng K.; Zhang H.; Mai L.; Pan M.; Mu S. Self-organized 3D porous graphene dual-doped with biomass-sponsored nitrogen and sulfur for oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2016, 8 (43), 29408–29418. 10.1021/acsami.6b08719. [DOI] [PubMed] [Google Scholar]
- He D.; Zhao W.; Li P.; Sun S.; Tan Q.; Han K.; Liu L.; Liu L.; Qu X. Bifunctional biomass-derived N, S dual-doped ladder-like porous carbon for supercapacitor and oxygen reduction reaction. J. Alloys Compd. 2019, 773, 11–20. 10.1016/j.jallcom.2018.09.141. [DOI] [Google Scholar]
- Zhang J.; Wang J.; Wu Z.; Wang S.; Wu Y.; Liu X. Heteroatom (nitrogen/sulfur)-doped graphene as an efficient electrocatalyst for oxygen reduction and evolution reactions. Catalysts 2018, 8, 475. 10.3390/catal8100475. [DOI] [Google Scholar]
- Song J.; Liu T. F.; Ali S.; Li B.; Su D. S. The synergy effect and reaction pathway in the oxygen reduction reaction on the sulfur and nitrogen dual doped graphene catalyst. Chem. Phys. Lett. 2017, 677, 65–69. 10.1016/j.cplett.2017.03.088. [DOI] [Google Scholar]
- Ma X. X.; He X. Q. Achieving superior performance for oxygen electrode catalyst by the assistance of NaCl to construct cobalt sulfide on nitrogen and sulfur dual-doped graphene. Int. J. of Hydrog. Energy 2018, 43, 13256–13265. 10.1016/j.ijhydene.2018.05.087. [DOI] [Google Scholar]
- Mathumba P.; Fernandes D. M.; Matos R.; Iwuoha E. I.; Freire C. Metal Oxide (Co3O4 and Mn3O4) Impregnation into S, N-doped Graphene for Oxygen Reduction Reaction (ORR). Materials 2020, 13, 1562. 10.3390/ma13071562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.; Wu T.; Wu Q.; Du S.; Chen D.; Chen B.; Chang M.; Luo X.; Liu Y. N-and S-co-doped graphene sheet-encapsulated Co9S8 nanomaterials as excellent electrocatalysts for the oxygen evolution reaction. J. Power Sources 2019, 417, 90–98. 10.1016/j.jpowsour.2019.02.024. [DOI] [Google Scholar]
- Ma R.; Lin G.; Zhou Y.; Liu Q.; Zhang T.; Shan G.; Yang M.; Wang J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 78. 10.1038/s41524-019-0210-3. [DOI] [Google Scholar]
- Jiang H.; Zhu Y.; Su Y.; Yao Y.; Liu Y.; Yang X.; Li C. Highly dual-doped multilayer nanoporous graphene: efficient metal-free electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 12642–12645. 10.1039/C5TA02792F. [DOI] [Google Scholar]
- Guruprasad K.; Maiyalagan T.; Shanmugam S. Phosphorus Doped MoS2 Nanosheet Promoted with Nitrogen, Sulfur Dual Doped Reduced Graphene Oxide as an Effective Electrocatalyst for Hydrogen Evolution. ACS Appl. Energy Mater. 2019, 2 (9), 6184–6194. 10.1021/acsaem.9b00629. [DOI] [Google Scholar]
- Cheng T.-Y.; Chou F.-P.; Huang S.-C.; Chang C.-Y.; Wu T.-K. Electroluminescence and photocatalytic hydrogen evolution of S, N co-doped graphene oxide quantum dots. J. Mater. Chem. A 2022, 10, 3650–3658. 10.1039/D1TA09917E. [DOI] [Google Scholar]
- Kumaran Y.; Maiyalagan T.; Yi S. C. An efficient CoMoS2 nanosheets on nitrogen, sulfur dual doped reduced graphene oxide as an electrocatalyst for the hydrogen evolution reaction. Int. J.Energy Res. 2021, 45, 17397–17407. 10.1002/er.5394. [DOI] [Google Scholar]
- Li M.; Zhou H.; Yang W.; Chen L.; Huang Z.; Zhang N.; Fu C.; Kuang Y. Co9S8 nanoparticles embedded in a N, S co-doped graphene-unzipped carbon nanotube composite as a high performance electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 1014–1021. 10.1039/C6TA08955K. [DOI] [Google Scholar]
- Li X.; Duan X.; Han C.; Fan X.; Li Y.; Zhang F.; Zhang G.; Peng W.; Wang S. Chemical activation of nitrogen and sulfur co-doped graphene as defect-rich carbocatalyst for electrochemical water splitting. Carbon 2019, 148, 540–549. 10.1016/j.carbon.2019.04.021. [DOI] [Google Scholar]
- Xie H.; Hou C.; Wang H.; Zhang Q.; Li Y. S, N co-doped graphene quantum dot/TiO2 composites for efficient photocatalytic hydrogen generation. Nanoscale Res. Lett. 2017, 12, 400. 10.1186/s11671-017-2101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayat A.; Saievar-Iranizad E. Vertically aligned rutile TiO2 nanorods sensitized with sulfur and nitrogen co-doped graphene quantum dots for water splitting: an energy level study. J. Alloys Compd. 2018, 755, 192–198. 10.1016/j.jallcom.2018.05.008. [DOI] [Google Scholar]
- Suryawanshi U. P.; Ghorpade U. V.; Lee D. M.; He M.; Shin S. W.; Kumar P. V.; Jang J. S.; Jung H. R.; Suryawanshi M. P.; Kim J. H. Colloidal Ni2P Nanocrystals Encapsulated in Heteroatom-Doped Graphene Nanosheets: A Synergy of 0D@2D Heterostructure Toward Overall Water Splitting. Chem. Mater. 2021, 33 (1), 234–245. 10.1021/acs.chemmater.0c03543. [DOI] [Google Scholar]
- Guo X.; Duan X.; Ji J.; Fan X.; Li Y.; Zhang F.; Zhang G.; Zhu Y. A.; Peng W.; Wang S. Synthesis of nitrogen and sulfur doped graphene on graphite foam for electro-catalytic phenol degradation and water splitting. J. Colloid Interface Sci. 2021, 583, 139–148. 10.1016/j.jcis.2020.09.053. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Li L.; Luo L.; Yang Y.; Yang Z.; Li H.; Zhou Y. Activation of persulfate with dual-doped reduced graphene oxide for degradation of alkylphenols. Chem. Eng. J. 2019, 376, 120891. 10.1016/j.cej.2019.01.170. [DOI] [Google Scholar]
- Tian Y.; Xu D.; Chu K.; Wei Z.; Liu W. Metal-free N, S co-doped graphene for efficient and durable nitrogen reduction reaction. J. Mater. Sci. 2019, 54, 9088–9097. 10.1007/s10853-019-03538-0. [DOI] [Google Scholar]
- Zhang X.; Zhu J.; Tiwary C. S.; Ma Z.; Huang H.; Zhang J.; Lu Z.; Huang W.; Wu Y. Palladium Nanoparticles Supported on Nitrogen and Sulfur Dual-Doped Graphene as Highly Active Electrocatalysts for Formic Acid and Methanol Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 10858–10865. 10.1021/acsami.6b01580. [DOI] [PubMed] [Google Scholar]
- An M.; Du L.; Du C.; Sun Y.; Wang Y.; Yin G.; Gao Y. Pt nanoparticles supported by sulfur and phosphorus co-doped graphene as highly active catalyst for acidic methanol electrooxidation. Electrochim. Acta 2018, 285, 202–213. 10.1016/j.electacta.2018.07.237. [DOI] [Google Scholar]
- Zhang K.; Chen X.; Wang L.; Zhang D.; Xue Z.; Zhou X.; Lu X. PtPd nanoparticles supported on sulfonated nitrogen sulfur co-doped graphene for methanol electro-oxidation. Int. J. Hydrog. Energy 2018, 43, 15931–15940. 10.1016/j.ijhydene.2018.06.157. [DOI] [Google Scholar]
- Zhang J. X.; Yang X. L.; Shao H. F.; Tseng C. C.; Wang D. S.; Tian S. S.; Hu W. J.; Jing C.; Tian J. N.; Zhao Y. C. Microwave-assisted Synthesis of Pd Oxide-rich Pd Particles on Nitrogen/Sulfur Co-Doped Graphene with Remarkably Enhanced Ethanol Electrooxidation. Fuel Cells 2017, 17, 115–122. 10.1002/fuce.201600153. [DOI] [Google Scholar]
- Mahyari M.; Bide Y.; Gavgani J. N. Iron (III) porphyrin supported on S and N co-doped graphene quantum dot as an efficient photocatalyst for aerobic oxidation of alcohols under visible light irradiation. Appl. Catal. A: Gen. 2016, 517, 100–109. 10.1016/j.apcata.2016.03.010. [DOI] [Google Scholar]
- Sajjadi S.; Khataee A.; Soltani R. D. C.; Hasanzadeh A. N, S co-doped graphene quantum dot–decorated Fe3O4 nanostructures: Preparation, characterization and catalytic activity. J. Phys. Chem. Solids 2019, 127, 140–150. 10.1016/j.jpcs.2018.12.014. [DOI] [Google Scholar]
- Rohani S.; Ziarani G. M.; Ziarati A.; Badiei A. Designer 3D CoAl-layered double hydroxide@N,S doped graphene hollow architecture decorated with Pd nanoparticles for Sonogashira couplings. Appl. Surf. Sci. 2019, 496, 143599. 10.1016/j.apsusc.2019.143599. [DOI] [Google Scholar]
- Rohani S.; Ziarani G. M.; Badiei A.; Ziarati A. Mesoporous hierarchically hollow flower-like CoAl-LDH@ N, S-doped Graphene@ Pd nanoarchitectures for heck couplings. Catal. Lett. 2019, 149, 2984–2993. 10.1007/s10562-019-02880-x. [DOI] [Google Scholar]
- Qu D.; Zheng M.; Du P.; Zhou Y.; Zhang L.; Li D.; Tan H.; Zhao Z.; Xie Z.; Sun Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. 10.1039/c3nr04402e. [DOI] [PubMed] [Google Scholar]
- Cai A.; Wang Q.; Chang Y.; Wang X. Graphitic carbon nitride decorated with S, N co-doped graphene quantum dots for enhanced visible-light-driven photocatalysis. J. Alloys Compd. 2017, 692, 183–189. 10.1016/j.jallcom.2016.09.030. [DOI] [Google Scholar]
- Tian H.; Shen K.; Hu X.; Qiao L.; Zheng W. N, S co-doped graphene quantum dots-graphene-TiO2 nanotubes composite with enhanced photocatalytic activity. J. Alloys Compd. 2017, 691, 369–377. 10.1016/j.jallcom.2016.08.261. [DOI] [Google Scholar]
- Zheng L.; Su H.; Zhang J.; Walekar L. S.; Molamahmood H. V.; Zhou B.; Long M.; Hu Y. H. Highly selective photocatalytic production of H2O2 on sulfur and nitrogen co-doped graphene quantum dots tuned TiO2. Appl. Catal. B: Environ. 2018, 239, 475–484. 10.1016/j.apcatb.2018.08.031. [DOI] [Google Scholar]
- Luo Y.; Li M.; Sun L.; Xu Y.; Hu G.; Tang T.; Wen J.; Li X. Tuning the photoluminescence of graphene quantum dots by co-doping of nitrogen and sulfur. J. Nanoparticle Res. 2017, 19, 363. 10.1007/s11051-017-4059-4. [DOI] [Google Scholar]
- Zhang R.; Adsetts J.R.; Nie Y.; Sun X.; Ding Z. Electrochemiluminescence of nitrogen-and sulfur-doped graphene quantum dots. Carbon 2018, 129, 45–53. 10.1016/j.carbon.2017.11.091. [DOI] [Google Scholar]
- Wang X. F.; Wang G. G.; Li J. B.; Liu Z.; Zhao W. F.; Han J.-C. Towards high-powered remote WLED based on flexible white-luminescent polymer composite films containing S, N co-doped graphene quantum dots. Chem. Eng. J. 2018, 336, 406–415. 10.1016/j.cej.2017.12.056. [DOI] [Google Scholar]
- Zhang B. X.; Gao H.; Li X. L. Synthesis and optical properties of nitrogen and sulfur co-doped graphene quantum dots. New J. Chem. 2014, 38, 4615–4621. 10.1039/C4NJ00965G. [DOI] [Google Scholar]
- Li J.; Yang S.; Wang G.; Huang T.; Guo Q.; Liu Z.; He P.; Zheng X.; Wang Y.; Xu A.; Zhao M.; Zhu W.; Chen D.; Ding G. Seed-Initiated Synthesis and Tunable Doping Graphene for High-Performance Photodetectors. Adv. Optical Mater. 2019, 7, 1901388. 10.1002/adom.201901388. [DOI] [Google Scholar]
- Adaikalam K.; Ramesh S.; Santhoshkumar P.; Kim H. S.; Park H. C.; Kim H. S. MnO2/Co3O4 with N and S co-doped graphene oxide bimetallic nanocomposite for hybrid supercapacitor and photosensor applications. Int. J. Energy Res. 2022, 46, 4494–4505. 10.1002/er.7443. [DOI] [Google Scholar]
- Schroer Z. S.; Wu Y.; Xing Y.; Wu X.; Liu X.; Wang Xu; Pino O. G.; Zhou C.; Combs C.; Pu Q.; Wu M.; Zhao J. X.; Chen J. Nitrogen–sulfur-doped graphene quantum dots with metal ion-resistance for bioimaging. ACS Appl. Nano Mater. 2019, 2 (11), 6858–6865. 10.1021/acsanm.9b01309. [DOI] [Google Scholar]
- Qu D.; Sun Z.; Zheng M.; Li J.; Zhang Y.; Zhang G.; Zhao H.; Liu X.; Xie Z. Three Colors Emission from S,N Co-doped Graphene Quantum Dots for Visible Light H2 Production and Bioimaging. Adv. Opt. Mater. 2015, 3, 360–367. 10.1002/adom.201400549. [DOI] [Google Scholar]
- Chen H.; Ma X.; Shen P. K. In-situ encapsulating FeS/Fe3C nanoparticles into nitrogen-sulfur dual-doped graphene networks for high-rate and ultra-stable lithium storage. J. Alloys Compd. 2019, 779, 193–201. 10.1016/j.jallcom.2018.11.295. [DOI] [Google Scholar]
- Li G.; He B.; Zhou M.; Wang G.; Zhou N.; Xu W.; Hou Z. Solvothermal Synthesis of Mesoporous Manganese Sulfide Nanoparticles Supported on Nitrogen and Sulfur Co-doped Graphene with Superior Lithium Storage Performance. ChemElectroChem. 2017, 4, 81–89. 10.1002/celc.201600327. [DOI] [Google Scholar]
- Gao X.; Wang B.; Zhang Y.; Liu H.; Liu H.; Wu H.; Dou S. Graphene-scroll-sheathed α-MnS coaxial nanocables embedded in N, S Co-doped graphene foam as 3D hierarchically ordered electrodes for enhanced lithium storage. Energy Storage Mater. 2019, 16, 46–55. 10.1016/j.ensm.2018.04.027. [DOI] [Google Scholar]
- Wang M.; Huang Y.; Chen X.; Wang K.; Wu H.; Zhang N.; Fu H. Synthesis of nitrogen and sulfur co-doped graphene supported hollow ZnFe2O4 nanosphere composites for application in lithium-ion batteries. J. Alloys. Compd. 2017, 691, 407–415. 10.1016/j.jallcom.2016.08.285. [DOI] [Google Scholar]
- Guo P.; Xiao F.; Liu Q.; Liu H.; Guo Y.; Gong J. R.; Wang S.; Liu Y. One-Pot Microbial Method to Synthesize Dual-Doped Graphene and Its Use as High-Performance Electrocatalyst. Sci. Rep 2013, 3, 3499. 10.1038/srep03499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees M.; Batool S.; Kong J.; Zhuang Q.; Liu H.; Shao Q.; Lu N.; Feng Y.; Wujcik E. K.; Gao Q.; Ding T.; Wei R.; Guo Z. Polyborosilazane derived ceramics Nitrogen sulfur dual doped graphene nanocomposite anode for enhanced lithium ion batteries. Electrochim. Acta 2019, 296, 925–937. 10.1016/j.electacta.2018.11.088. [DOI] [Google Scholar]
- Song X.; Ma X.; Ning G.; Gao D.; Yu Z.; Xiao Z. The orientation construction of S and N dual-doped discoid-like graphene with high-rate electrode property. Appl. Surf. Sci. 2018, 442, 467–475. 10.1016/j.apsusc.2018.01.315. [DOI] [Google Scholar]
- Kotal M.; Kim J.; Kim K. J.; Oh I. K. Sulfur and nitrogen co-doped graphene electrodes for high-performance ionic artificial muscles. Adv. Mater. 2016, 28, 1610–1615. 10.1002/adma.201505243. [DOI] [PubMed] [Google Scholar]
- Huo J.; Ren Y.; Xue Y.; Liu Y.; Guo S. Sulfur/nitrogen dual-doped three-dimensional reduced graphene oxide modified with mesoporous TiO2 nanoparticles for promising lithium-ion battery anodes. J. Alloys Compd. 2021, 868, 159183. 10.1016/j.jallcom.2021.159183. [DOI] [Google Scholar]
- Kim J.-H.; Kannan A. G.; Woo H.-S.; Jin D.-G.; Kim W.; Ryu K.; Kim D.-W. A bi-functional metal-free catalyst composed of dual-doped graphene and mesoporous carbon for rechargeable lithium–oxygen batteries. J. Mater. Chem. A 2015, 3, 18456–18465. 10.1039/C5TA05334J. [DOI] [Google Scholar]
- Jang S.; Kim J.; Na E.; Song M.; Choi J.; Song K. H.; Baeck S. H.; Shim S. E. Facile synthesis of mesoporous and highly nitrogen/sulfur dual-doped graphene and its ultrahigh discharge capacity in non-aqueous lithium oxygen batteries. Carbon Lett. 2019, 29, 297–305. 10.1007/s42823-019-00026-y. [DOI] [Google Scholar]
- Zeng X.; You C.; Leng L.; Dang D.; Qiao X.; Li X.; Li Y.; Liao S.; Adzic R. R. Ruthenium nanoparticles mounted on multielement co-doped graphene: an ultra-high-efficiency cathode catalyst for Li–O2 batteries. J. Mater. Chem. A 2015, 3, 11224–11231. 10.1039/C5TA01887K. [DOI] [Google Scholar]
- Chen D.; Yang R.; Chen L.; Zou Y.; Ren B.; Li L.; Li S.; Yan Y.; Xu Y. One-pot fabrication of nitrogen and sulfur dual-doped graphene/sulfur cathode via microwave assisted method for long cycle-life lithium-sulfur batteries. J. Alloys Compd. 2018, 746, 116–124. 10.1016/j.jallcom.2018.02.279. [DOI] [Google Scholar]
- Li X.; Yu Y.; Tang Z.; Yang Y.; Li Y.; Cao J.; Chen L. N,S-doped graphene derived from graphene oxide and thiourea-formaldehyde resin for high stability lithium–sulfur batteries. Phys. Chem. Chem. Phys. 2022, 24, 2879–2886. 10.1039/D1CP04675F. [DOI] [PubMed] [Google Scholar]
- Ci H.; Wang M.; Sun Z.; Wei C.; Cai J.; Lu C.; Cui G.; Liu Z.; Sun J. Direct insight into sulfiphilicity-lithiophilicity design of bifunctional heteroatom-doped graphene mediator toward durable Li-S batteries. J. Energy Chem. 2022, 66, 474–482. 10.1016/j.jechem.2021.08.048. [DOI] [Google Scholar]
- Pang Q.; Tang J.; Huang H.; Liang X.; Hart C.; Tam K. C.; Nazar L. F. A nitrogen and sulfur dual-doped carbon derived from Polyrhodanine@Cellulose for advanced lithium–sulfur batteries. Adv. Mater. 2015, 27, 6021–6028. 10.1002/adma.201502467. [DOI] [PubMed] [Google Scholar]
- Balach J.; Singh H. K.; Gomoll S.; Jaumann T.; Klose M.; Oswald S.; Richter M.; Eckert J.; Giebeler L. Synergistically enhanced polysulfide chemisorption using a flexible hybrid separator with N and S dual-doped mesoporous carbon coating for advanced lithium–sulfur. ACS Appl. Mater. Interfaces 2016, 8 (23), 14586–14595. 10.1021/acsami.6b03642. [DOI] [PubMed] [Google Scholar]
- Li N.; Gan F.; Wang P.; Chen K.; Chen S.; He X. In situ synthesis of 3D sulfur-doped graphene/sulfur as a cathode material for lithium-sulfur batteries. J. Alloys Compd. 2018, 754, 64–71. 10.1016/j.jallcom.2018.04.018. [DOI] [Google Scholar]
- Zhou Y.; Zeng Y.; Xu D.; Li P.; Wang H.-G.; Li X.; Li Y.; Wang Y. Nitrogen and sulfur dual-doped graphene sheets as anode materials with superior cycling stability for lithium-ion batteries. Electrochim. Acta 2015, 184, 24–31. 10.1016/j.electacta.2015.10.026. [DOI] [Google Scholar]
- Shan H.; Li X.; Cui Y.; Xiong D.; Yan B.; Li D.; Lushington A.; Sun X. Sulfur/nitrogen dual-doped porous graphene aerogels enhancing anode performance of lithium ion batteries. Electrochim. Acta 2016, 205, 188–197. 10.1016/j.electacta.2016.04.105. [DOI] [Google Scholar]
- Feng Q.; Li T.; Sui Y.; Xiao B.; Wang T.; Sun Z.; Qi J.; Wei F.; Meng Q.; Ren Y.; Xue X. Facile synthesis and first-principles study of nitrogen and sulfur dual-doped porous graphene aerogels/natural graphite as anode materials for Li-ion batteries. J. Alloys Compd. 2021, 884, 160923. 10.1016/j.jallcom.2021.160923. [DOI] [Google Scholar]
- Wang C.; Zhang X.; Qian Y.; Wu H.; Kan E. First-principles study on S and N doping graphene/SnS2 heterostructure for lithium-ion battery. Chem. Phys. Lett. 2021, 769, 138391. 10.1016/j.cplett.2021.138391. [DOI] [Google Scholar]
- Zhu J.; Tu W.; Pan H.; Zhang H.; Liu B.; Cheng Y.; Deng Z.; Zhang H. Self-Templating Synthesis of Hollow Co3O4 Nanoparticles Embedded in N,S-Dual-Doped Reduced Graphene Oxide for Lithium Ion Batteries. ACS Nano 2020, 14 (5), 5780–5787. 10.1021/acsnano.0c00712. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Qian K.; Lv J.; Yan W.; Liu J.; Ai J.; Zhang Y.; Guo T.; Zhou Z.; Xu S.; Guo Z. Encapsulation of Fe3O4 Nanoparticles into N, S co-Doped Graphene Sheets with Greatly Enhanced Electrochemical Performance. Sci. Rep. 2016, 6, 27957. 10.1038/srep27957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D.; Wang C.; Shi C.; Tan N. Facile synthesis of N and S co-doped graphene sheets as anode materials for high-performance lithium-ion batteries. J. Alloys Compd. 2018, 731, 235–242. 10.1016/j.jallcom.2017.10.043. [DOI] [Google Scholar]
- Li R.; Li J.; Qi K.; Ge X.; Zhang Q.; Zhang B. One-step synthesis of 3D sulfur/nitrogen dual-doped graphene supported nano silicon as anode for Li-ion batteries. Appl. Surf. Sci. 2018, 433, 367–373. 10.1016/j.apsusc.2017.10.061. [DOI] [Google Scholar]
- Miao X.; Sun D.; Zhou X.; Lei Z. Designed formation of nitrogen and sulfur dual-doped hierarchically porous carbon for long-life lithium and sodium ion batteries. Chem. Eng. J. 2019, 364, 208–216. 10.1016/j.cej.2019.01.158. [DOI] [Google Scholar]
- Wang H.; Jiang C.; Yuan C.; Wu Q.; Li Q.; Duan Q. Complexing agent engineered strategy for anchoring SnO2 nanoparticles on sulfur/nitrogen co-doped graphene for superior lithium and sodium ion storage. Chem. Eng. J. 2018, 332, 237–244. 10.1016/j.cej.2017.09.081. [DOI] [Google Scholar]
- Liu X.; Hao Y.; Shu J.; Sari H. M. K.; Lin L.; Kou H.; Li J.; Liu W.; Yan B.; Li D.; Zhang J.; Li X. Li, Nitrogen/sulfur dual-doping of reduced graphene oxide harvesting hollow ZnSnS3 nano-microcubes with superior sodium storage. Nano Energy 2019, 57, 414–423. 10.1016/j.nanoen.2018.12.024. [DOI] [Google Scholar]
- Jiang Y.; Wu Y.; Chen Y.; Qi Z.; Shi J.; Gu L.; Yu Y. Design Nitrogen (N) and Sulfur (S) Co-Doped 3D Graphene Network Architectures for High-Performance Sodium Storage. Small 2018, 14, 1703471. 10.1002/smll.201703471. [DOI] [PubMed] [Google Scholar]
- Bag S.; Roy A.; Mitra S. Sulfur, Nitrogen Dual Doped Reduced Graphene Oxide Supported Two-Dimensional Sb2S3 Nanostructures for the Anode Material of Sodium-Ion Battery. ChemistrySelect 2019, 4, 6679–6686. 10.1002/slct.201901153. [DOI] [Google Scholar]
- Xu X.; Zeng H.; Han D.; Qiao K.; Xing W.; Rood M. J.; Yan Z. Nitrogen and sulfur co-doped graphene nanosheets to improve anode materials for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 37172–37180. 10.1021/acsami.8b15940. [DOI] [PubMed] [Google Scholar]
- Liang X.; Ou X.; Zheng F.; Pan Q.; Xiong X.; Hu R.; Yang C.; Liu M. Surface Modification of Na3V2(PO4)3 by Nitrogen and Sulfur Dual-Doped Carbon Layer with Advanced Sodium Storage Property. ACS Appl. Mater. Interfaces 2017, 9 (15), 13151–13162. 10.1021/acsami.7b00818. [DOI] [PubMed] [Google Scholar]
- Lu C.; Sun Z.; Yu L.; Lian X.; Yi Y.; Li J.; Liu Z.; Dou S.; Sun J. Enhanced kinetics harvested in heteroatom dual-doped graphitic hollow architectures toward high rate printable potassium-ion batteries. Adv. Energy Mater. 2020, 10, 2001161. 10.1002/aenm.202001161. [DOI] [Google Scholar]
- Yang W.; Zhou J.; Wang S.; Wang Z.; Lv F.; Zhang W.; Zhang W.; Sun Q.; Guo S. A three-dimensional carbon framework constructed by N/S co-doped graphene nanosheets with expanded interlayer spacing facilitates potassium ion storage. ACS Energy Lett. 2020, 5 (5), 1653–1661. 10.1021/acsenergylett.0c00413. [DOI] [Google Scholar]
- Geng D.; Ding N. N.; Hor T. S. A.; Chien S. W.; Liu Z.; Zong Y. Cobalt sulfide nanoparticles impregnated nitrogen and sulfur co-doped graphene as bifunctional catalyst for rechargeable Zn–air batteries. RSC Adv. 2015, 5, 7280–7284. 10.1039/C4RA13404D. [DOI] [Google Scholar]
- Ganesan P.; Ramakrishnan P.; Prabu M.; Shanmugam S. Nitrogen and sulfur Co-doped graphene supported cobalt sulfide nanoparticles as an efficient air cathode for zinc-air battery. Electrochim. Acta 2015, 183, 63–69. 10.1016/j.electacta.2015.05.182. [DOI] [Google Scholar]
- Chen S.; Duan J.; Zheng Y.; Chen X.; Du X. W.; Jaroniec M.; Qiao S.-Z. Ionic liquid-assisted synthesis of N/S-double doped graphene microwires for oxygen evolution and Zn–air batteries. Energy Storage Mater. 2015, 1, 17–24. 10.1016/j.ensm.2015.08.001. [DOI] [Google Scholar]
- Zhang J.; Zhou H.; Zhu J.; Hu P.; Hang C.; Yang J.; Peng T.; Mu S.; Huang Y. Facile synthesis of defect-rich and S/N co-doped graphene-like carbon nanosheets as an efficient electrocatalyst for primary and all-solid-state Zn–air batteries. ACS Appl. Mater. Interfaces 2017, 9 (29), 24545–24554. 10.1021/acsami.7b04665. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Wang H.; Liu F.; Gai H.; Ji S.; Linkov V.; Wang R. Hydrophobic 3D Fe/N/S doped graphene network as oxygen electrocatalyst to achieve unique performance of zinc-air battery. Chem. Eng. J. 2018, 353, 472–480. 10.1016/j.cej.2018.07.140. [DOI] [Google Scholar]
- Shao Q.; Liu J.; Wu Q.; Li Q.; Wang H.; Li Y.; Duan Q. In situ coupling strategy for anchoring monodisperse Co9S8 nanoparticles on S and N dual-doped graphene as a bifunctional electrocatalyst for rechargeable Zn–Air Battery. Nano-Micro Lett. 2019, 11, 4. 10.1007/s40820-018-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.; Gong M.; Zhou Y.; Fang J.; Huang L.; et al. Sulphur modulated Ni3FeN supported on N/S co-doped graphene boosts rechargeable/flexible Zn-air battery performance. Appl. Catal., B 2020, 274, 119086. 10.1016/j.apcatb.2020.119086. [DOI] [Google Scholar]
- Zhao Z.; Xia Z. Interactions between Dopants in Dual-Doped Graphene Nanoribbons as Metal-Free Bifunctional Catalysts for Fuel Cell and Metal-Air Batteries. MRS Advances 2016, 1, 421–425. 10.1557/adv.2016.32. [DOI] [Google Scholar]
- Li Q.; Bai A.; Xue Z.; Zheng Y.; Sun H. Nitrogen and sulfur co-doped graphene composite electrode with high electrocatalytic activity for vanadium redox flow battery application. Electrochim. Acta 2020, 362, 137223. 10.1016/j.electacta.2020.137223. [DOI] [Google Scholar]
- Daugherty M. C.; Gu S.; Aaron D. S.; Mallick B. C.; Gandomi Y. A.; Hsieh C. T. Decorating sulfur and nitrogen co-doped graphene quantum dots on graphite felt as high-performance electrodes for vanadium redox flow batteries. J. Power Sources 2020, 477, 228709. 10.1016/j.jpowsour.2020.228709. [DOI] [Google Scholar]
- Li Z.; He W.; Wang X.; Wang X.; Song M.; Zhao J. N/S dual-doped graphene with high defect density for enhanced supercapacitor properties. Int. J. Hydrog. Energy 2020, 45, 112–122. 10.1016/j.ijhydene.2019.10.196. [DOI] [Google Scholar]
- Xing L. B.; Hou S. F.; Zhang J. L.; Zhou J.; Li Z.; Si W.; Zhuo S. A facile preparation of three dimensional N, S co-doped graphene hydrogels with thiocarbohydrazide for electrode materials in supercapacitor. Mater. Lett. 2015, 147, 97–100. 10.1016/j.matlet.2015.02.031. [DOI] [Google Scholar]
- Chen W.; Shi J.; Zhu T.; Wang Q.; Qiao J.; Zhang J. Preparation of nitrogen and sulfur dual-doped mesoporous carbon for supercapacitor electrodes with long cycle stability. Electrochim. Acta 2015, 177, 327–334. 10.1016/j.electacta.2015.01.093. [DOI] [Google Scholar]
- Tran N. Q.; Kang B. K.; Woo M. H.; Yoon D. H. Enrichment of Pyrrolic Nitrogen by Hole Defects in Nitrogen and Sulfur Co-Doped Graphene Hydrogel for Flexible Supercapacitors. ChemSusChem 2016, 9, 2261–2268. 10.1002/cssc.201600668. [DOI] [PubMed] [Google Scholar]
- Wang T.; Wang L. X.; Wu D. L.; Xia W.; Jia D. Z. Interaction between nitrogen and sulfur in co-doped graphene and synergetic effect in supercapacitor. Sci. Rep. 2015, 5, 9591. 10.1038/srep09591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poompiew N.; Pattananuwat P.; Potiyaraj P. In situ hydrothermal synthesis of nickel cobalt sulfide nanoparticles embedded on nitrogen and sulfur dual doped graphene for a high performance supercapacitor electrode. RSC Adv. 2021, 11, 25057–25067. 10.1039/D1RA03607F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. Q.; Shan Y. Q.; Wang M. Y.; Alothman Z. A.; Xu Z.-X; Duan P. G.; Zhou J.; Luque R. Mechanochemical Preparation of N,S-Doped Graphene Oxide Using (NH4)2SO4 for Supercapacitor Applications. ACS Sustainable Chem. Eng. 2020, 8 (51), 18810–18815. 10.1021/acssuschemeng.0c05918. [DOI] [Google Scholar]
- Lu Z.; Xu X.; Chen Y.; Wang X.; Sun L.; Zhuo K. Nitrogen and sulfur co-doped graphene aerogel with hierarchically porous structure for high-performance supercapacitors. Green Energy Environ. 2020, 5, 69–75. 10.1016/j.gee.2019.06.001. [DOI] [Google Scholar]
- Zhang L.; Chen H.; Lu X.; Wang Y.; Tan L.; Sui D.; Qi W. Fabrication of N, S co-doped graphene aerogel for high-performance supercapacitors: π-conjugated planar molecules as efficient dopants and pillared agents. Appl. Surf. Sci. 2020, 529, 147022. 10.1016/j.apsusc.2020.147022. [DOI] [Google Scholar]
- Gopalsamy K.; Balamurugan J.; Thanh T. D.; Kim N. H.; Lee J. H. Fabrication of nitrogen and sulfur co-doped graphene nanoribbons with porous architecture for high-performance supercapacitors. Chem. Eng. J. 2017, 312, 180–190. 10.1016/j.cej.2016.11.130. [DOI] [Google Scholar]
- Tian P.; Zang J.; Jia S.; Zhang Y.; Gao H.; Zhou S.; Wang W.; Xu H.; Wang Y. Preparation of S/N co-doped graphene through a self-generated high gas pressure for high rate supercapacitor. Appl. Surf. Sci. 2018, 456, 781–788. 10.1016/j.apsusc.2018.06.213. [DOI] [Google Scholar]
- Domga; Oladoyinbo F.; Noumi G. B.; Tchatchueng J. B.; Sieliechi M. S.; Sathish M.; Pattanayak D. P.; Karnan M. A simple, economical one-pot microwave assisted synthesis of nitrogen and sulfur co-doped graphene for high energy supercapacitors. Electrochim. Acta 2020, 341, 135999. 10.1016/j.electacta.2020.135999. [DOI] [Google Scholar]
- Liu J.; Chen X.; Zhu Y.; Chen R.; Yuan W. NiCo2S4/nitrogen and sulfur dual-doped three-dimensional holey-reduced graphene oxide composite architectures as high-rate battery-type cathode materials for hybrid supercapacitors. Vacuum 2021, 190, 110302. 10.1016/j.vacuum.2021.110302. [DOI] [Google Scholar]
- Kan Y.; Ma X.; Ning G.; Li Y.; Zhou Y. S, N Dual-Doped Graphene Fibers: A High-Performance Electrode Material for Supercapacitors. ChemElectroChem. 2017, 4, 2677–2682. 10.1002/celc.201700406. [DOI] [Google Scholar]
- Zhang D.; Lei L.; Shang Y.; Wang K.; Wang Y. The composite capacitive behaviors of the N and S dual doped ordered mesoporous carbon with ultrahigh doping level. Appl. Surf. Sci. 2016, 360, 807–815. 10.1016/j.apsusc.2015.11.071. [DOI] [Google Scholar]
- Li Z.; Wang X.; Xu M.; Yin Z.; Tan X.; Zhao J. Facile Synthesis and Outstanding Supercapacitor Performance of Ternary Nanocomposite of Silver Particles Decorated N/S Dual-Doped Graphene and MoS2 Microspheres Stabilized by Graphene. J. Electrochem. Soc. 2022, 169, 020525 10.1149/1945-7111/ac4f75. [DOI] [Google Scholar]
- Li Z.; Wang X.; Yin Z.; Zhao J.; Song M.; Wu Z.; Li H.; Wang X. Ag nanoparticles decorated N/S dual-doped graphene nanohybrids for high-performance asymmetric supercapacitors. Carbon 2020, 161, 726–735. 10.1016/j.carbon.2020.02.013. [DOI] [Google Scholar]
- Li Z.; Zhao J.; Yin Z.; Wang X.; Wu Z.; Liu D.; Zhu M.; Wang X. Novel Robust Nanohybrids of Pompon-like MoS2 and N/S Dual-Doped Graphene for High-Performance Asymmetric Supercapacitors. Energy Fuels 2021, 35 (3), 2692–2703. 10.1021/acs.energyfuels.0c03403. [DOI] [Google Scholar]
- Jia S.; Wei J.; Meng X.; Shao Z. Facile and friendly preparation of N/S Co-doped graphene-like carbon nanosheets with hierarchical pore by molten salt for all-solid-state supercapacitor. Electrochim. Acta 2020, 331, 135338. 10.1016/j.electacta.2019.135338. [DOI] [Google Scholar]
- Wu X.; Ding B.; Zhang C.; Li B.; Fan Z. Self-activation of nitrogen and sulfur dual-doping hierarchical porous carbons for asymmetric supercapacitors with high energy densities. Carbon 2019, 153, 225–233. 10.1016/j.carbon.2019.07.020. [DOI] [Google Scholar]
- Ma L.; Liu J.; Lv S.; Zhou Q.; Shen X.; Mo S.; Tong H. Scalable one-step synthesis of N, S co-doped graphene-enhanced hierarchical porous carbon foam for high-performance solid-state supercapacitors. J. Mater. Chem. A 2019, 7, 7591–7603. 10.1039/C9TA00038K. [DOI] [Google Scholar]
- Wang W.; Zhang W.; Wang G.; Li C. Electrophoresis-microwave synthesis of S, N-doped graphene foam for high-performance supercapacitors. J. Mater. Chem. A 2021, 9, 15766–15775. 10.1039/D1TA02877D. [DOI] [Google Scholar]
- Cheng L.; Hu Y.; Qiao D.; Zhu Y.; Wang H.; Jiao Z. One-step radiolytic synthesis of heteroatom (N and S) co-doped graphene for supercapacitors. Electrochim. Acta 2018, 259, 587–597. 10.1016/j.electacta.2017.11.022. [DOI] [Google Scholar]
- Wang Y.; Zhang M.; Pan D.; Li Y.; Ma T.; Xie J. Nitrogen/sulfur co-doped graphene networks uniformly coupled N-Fe2O3 nanoparticles achieving enhanced supercapacitor performance. Electrochim. Acta 2018, 266, 242–253. 10.1016/j.electacta.2018.02.040. [DOI] [Google Scholar]
- Chen L.; Li X.; Ma C.; Wang M.; Zhou J. Interaction and quantum capacitance of nitrogen/sulfur co-doped graphene: a theoretical calculation. J. Phys. Chem. C 2017, 121 (34), 18344–18350. 10.1021/acs.jpcc.7b04551. [DOI] [Google Scholar]
- Kannan A. G.; Samuthirapandian A.; Kim D. W. Electric double layer capacitors employing nitrogen and sulfur co-doped, hierarchically porous graphene electrodes with synergistically enhanced performance. J. Power Sources 2017, 337, 65–72. 10.1016/j.jpowsour.2016.10.109. [DOI] [Google Scholar]
- Luo Q.; Hao F.; Wang S.; Shen H.; Zhao L.; Li J.; Gratzel M.; Lin H. Highly efficient metal-free sulfur-doped and nitrogen and sulfur dual-doped reduced graphene oxide counter electrodes for dye-sensitized solar cells. J. Phys. Chem. C 2014, 118 (30), 17010–17018. 10.1021/jp5004424. [DOI] [Google Scholar]
- Xu X.; Yang W.; Li Y.; Tu Z.; Wu S.; Ma X.; Yang F.; Zhang L.; Chen S.; Wang A.; Liu H.; Richard P. Heteroatom-doped graphene-like carbon films prepared by chemical vapour deposition for bifacial dye-sensitized solar cells. Chem. Eng. J. 2015, 267, 289–296. 10.1016/j.cej.2015.01.028. [DOI] [Google Scholar]
- Raji Karunagaran J.; Janakiraman M.; Jonna N.; Natesan B.; Nallamuthu P. A PDDA functionalized nitrogen and sulphur doped graphene composite as the counter electrode for dye-sensitized solar cells. New J. Chem. 2018, 42, 10184–10190. 10.1039/C8NJ00850G. [DOI] [Google Scholar]
- Wang G.; Dong W.; Ma P.; Yan C.; Zhang W.; Liu J. Interconnected nitrogen and sulfur co-doped graphene-like porous carbon nanosheets with high electrocatalytic activity as counter electrodes for dye-sensitized and Electrochim. Acta 2018, 290, 273–281. 10.1016/j.electacta.2018.09.063. [DOI] [Google Scholar]
- Di Y.; Xiao Z.; Yan X.; Ru G.; Chen B.; Feng J. Nitrogen and sulfur dual-doped chitin-derived carbon/graphene composites as effective metal-free electrocatalysts for dye sensitized solar cells. Appl. Surf. Sci. 2018, 441, 807–815. 10.1016/j.apsusc.2018.02.071. [DOI] [Google Scholar]
- Kannan A. G.; Zhao J.; Jo S. G.; Kang Y. S.; Kim D. W. Nitrogen and sulfur co-doped graphene counter electrodes with synergistically enhanced performance for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 12232–12239. 10.1039/C4TA01927J. [DOI] [Google Scholar]
- Liu Y.; Wang Y.; Zheng X.; Wang Y. Synergistic effect of nitrogen and sulfur co-doped graphene as efficient metal-free counter electrode for dye-sensitized solar cells: a first-principle study. Comput. Mater. Sci. 2017, 136, 44–51. 10.1016/j.commatsci.2017.04.029. [DOI] [Google Scholar]
- Chen H.; Luo Q.; Liu T.; Tai M.; Lin J.; Murugadoss V.; Lin H.; Wang J.; Guo Z.; Wang N. Boosting multiple interfaces by co-doped graphene quantum dots for high efficiency and durability perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12 (12), 13941–13949. 10.1021/acsami.9b23255. [DOI] [PubMed] [Google Scholar]
- Zhao T.; Yang D.; Xu T.; Zhang M.; Zhang S.; Qin L.; Yu Z.-Z. Cold-Resistant Nitrogen/Sulfur Dual-Doped Graphene Fiber Supercapacitors with Solar–Thermal Energy Conversion Effect. Chem.—Eur. J. 2021, 27, 3473–3482. 10.1002/chem.202004703. [DOI] [PubMed] [Google Scholar]
- Majumder T.; Dhar S.; Debnath K.; Mondal S. P. Role of S, N co-doped graphene quantum dots as a green photosensitizer with Ag-doped ZnO nanorods for improved electrochemical solar energy conversion. Mater. Res. Bull. 2017, 93, 214–222. 10.1016/j.materresbull.2017.05.004. [DOI] [Google Scholar]
- Li M.; Guo Q.; Xie J.; Li Y.; Feng Y. CuO nanoparticles supported on nitrogen and sulfur co-doped graphene nanocomposites for non-enzymatic glucose sensing. J. Nanoparticle Res. 2017, 19, 11. 10.1007/s11051-016-3712-7. [DOI] [Google Scholar]
- Chen G.; Liu Y.; Liu Y.; Tian Y.; Zhang X. Nitrogen and sulfur dual-doped graphene for glucose biosensor application. J. Electroanal. Chem. 2015, 738, 100–107. 10.1016/j.jelechem.2014.11.020. [DOI] [Google Scholar]
- Tian Y.; Ma Y.; Liu H.; Zhang X.; Peng W. One-step and rapid synthesis of nitrogen and sulfur co-doped graphene for hydrogen peroxide and glucose sensing. J. Electroanal. Chem. 2015, 742, 8–14. 10.1016/j.jelechem.2015.01.029. [DOI] [Google Scholar]
- Masteri-Farahani M.; Ghorbani F.; Mosleh N. Boric acid modified S and N co-doped graphene quantum dots as simple and inexpensive turn-on fluorescent nanosensor for quantification of glucose. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 245, 118892. 10.1016/j.saa.2020.118892. [DOI] [PubMed] [Google Scholar]
- Varodi C.; Pogăcean F.; Cioriţă A.; Pană O.; Leoştean C.; Cozar B.; Radu T.; Coros M.; Stefan-van-Staden R. L.; Pruneanu S. M. Nitrogen and sulfur co-doped graphene as efficient electrode material for l-cysteine detection. Chemosensors 2021, 9, 146. 10.3390/chemosensors9060146. [DOI] [Google Scholar]
- Qi Y.; Cao Y.; Meng X.; Cao J.; Li X.; Hao Q.; Lei W.; Li Q.; Li J.; Si W. Facile synthesis of 3D sulfur/nitrogen co-doped graphene derived from graphene oxide hydrogel and the simultaneous determination of hydroquinone and catechol. Sens. Actuators B: Chem. 2019, 279, 170–176. 10.1016/j.snb.2018.09.067. [DOI] [Google Scholar]
- Chen C.; Zhao D.; Hu T.; Sun J.; Yang X. Highly fluorescent nitrogen and sulfur co-doped graphene quantum dots for an inner filter effect-based cyanide sensor. Sens. Actuators B: Chem. 2017, 241, 779–788. 10.1016/j.snb.2016.11.010. [DOI] [Google Scholar]
- Lv H.; Zhang X.; Li Y.; Ren Y.; Zhang C.; Wang P.; Xu Z.; Li X.; Chen Z.; Dong Y. An electrochemical sandwich immunosensor for cardiac troponin I by using nitrogen/sulfur co-doped graphene oxide modified with Au@Ag nanocubes as amplifiers. Microchim. Acta 2019, 186, 416. 10.1007/s00604-019-3526-2. [DOI] [PubMed] [Google Scholar]
- Peng J.; Zhao Z.; Zheng M.; Su B.; Chen X.; Chen X. Electrochemical synthesis of phosphorus and sulfur co-doped graphene quantum dots as efficient electrochemiluminescent immunomarkers for monitoring okadaic acid. Sens. Actuators B: Chem. 2020, 304, 127383. 10.1016/j.snb.2019.127383. [DOI] [Google Scholar]
- Liu Z.; Xiao J.; Wu X.; Lin L.; Weng S.; Chen M.; Cai X.; Lin X. Switch-on fluorescent strategy based on N and S co-doped graphene quantum dots (NS/GQDs) for monitoring pyrophosphate ions in synovial fluid of arthritis patients. Sens. Actuators B: Chem. 2016, 229, 217–224. 10.1016/j.snb.2016.01.127. [DOI] [Google Scholar]
- Safardoust-Hojaghan H.; Amiri O.; Hassanpour M.; Panahi-Kalamuei M.; Moayedi H.; Salavati-Niasari M. S, N co-doped graphene quantum dots-induced ascorbic acid fluorescent sensor: Design, characterization and performance. Food Chem. 2019, 295, 530–536. 10.1016/j.foodchem.2019.05.169. [DOI] [PubMed] [Google Scholar]
- Yasmin S.; Ahmed M. S.; Jeon S. Determination of dopamine by dual doped graphene-Fe2O3 in presence of ascorbic acid. J. Electrochem. Soc. 2015, 162, B363. 10.1149/2.0751514jes. [DOI] [Google Scholar]
- Zhang K.; Chen X.; Li Z.; Wang Y.; Sun S.; Guo T.; Zhang D.; Xue Z.; Zhou X.; Lu X.; Wang L. Au-Pt bimetallic nanoparticles decorated on sulfonated nitrogen sulfur co-doped graphene for simultaneous determination of dopamine and uric acid. Talanta 2018, 178, 315–323. 10.1016/j.talanta.2017.09.047. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Wu J.; Han L.; Wang X.; Li W.; Guo H.; Wei H. Nanozyme sensor arrays based on heteroatom-doped graphene for detecting pesticides. Anal. Chem. 2020, 92, 7444–7452. 10.1021/acs.analchem.9b05110. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Gao J.; Song H.; Lin X.; Zhang S. Nature of the synergistic effect of N and S Co-Doped graphene for the enhanced simultaneous determination of toxic pollutants. ACS Appl. Mater. Interfaces 2019, 11 (47), 44545–44555. 10.1021/acsami.9b13211. [DOI] [PubMed] [Google Scholar]
- Nemati F.; Hosseini M.; Zare-Dorabei R.; Ganjali M. R. Sensitive recognition of ethion in food samples using turn-on fluorescence N and S co-doped graphene quantum dots. Anal. Methods 2018, 10, 1760–1766. 10.1039/C7AY02850D. [DOI] [Google Scholar]
- Wang W.; Xu S.; Li N.; Huang Z.; Su B.; Chen X. Sulfur and phosphorus co-doped graphene quantum dots for fluorescent monitoring of nitrite in pickles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 221, 117211. 10.1016/j.saa.2019.117211. [DOI] [PubMed] [Google Scholar]
- Martins E. C.; Santana E. R.; Spinelli A. Nitrogen and sulfur co-doped graphene quantum dot-modified electrode for monitoring of multivitamins in energy drinks. Talanta 2023, 252, 123836. 10.1016/j.talanta.2022.123836. [DOI] [PubMed] [Google Scholar]
- Jlassi K.; Mallick S.; Eribi A.; Chehimi M. M.; Ahmad Z.; Touati F.; Krupa I. Facile preparation of NS co-doped graphene quantum dots (GQDs) from graphite waste for efficient humidity sensing. Sens. Actuators B: Chem. 2021, 328, 129058. 10.1016/j.snb.2020.129058. [DOI] [Google Scholar]
- Gavgani J. N.; Hasani A.; Nouri M.; Mahyari M.; Salehi A. Highly sensitive and flexible ammonia sensor based on S and N co-doped graphene quantum dots/polyaniline hybrid at room temperature. Sens. Actuators B 2016, 229, 239–248. 10.1016/j.snb.2016.01.086. [DOI] [Google Scholar]
- Mondal T. K.; Dinda D.; Saha S. K. Nitrogen, sulphur co-doped graphene quantum dot: An excellent sensor for nitroexplosives. Sens. Actuators B: Chem. 2018, 257, 586–596. 10.1016/j.snb.2017.11.012. [DOI] [Google Scholar]
- Zhang R.; Zhang C.; Zheng F.; Li X.; Sun C. L.; Chen W. Nitrogen and sulfur co-doped graphene nanoribbons: a novel metal-free catalyst for high performance electrochemical detection of 2, 4, 6-trinitrotoluene (TNT). Carbon 2018, 126, 328–337. 10.1016/j.carbon.2017.10.042. [DOI] [Google Scholar]
- Boonta W.; Talodthaisong C.; Sattayaporn S.; Chaicham C.; Chaicham A.; Sahasithiwat S.; Kangkaew L.; Kulchat S. The synthesis of nitrogen and sulfur co-doped graphene quantum dots for fluorescence detection of cobalt (II) ions in water. Mater. Chem. Front. 2020, 4, 507–516. 10.1039/C9QM00587K. [DOI] [Google Scholar]
- Qu C.; Zhang D.; Yang R.; Hu J.; Qu L. Nitrogen and sulfur co-doped graphene quantum dots for the highly sensitive and selective detection of mercury ion in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 588–596. 10.1016/j.saa.2018.07.097. [DOI] [PubMed] [Google Scholar]
- Gu S.; Hsieh C. T.; Tsai Y. Y.; Gandomi Y. A.; Yeom S.; Kihm K. D.; Fu C. C.; Juang R. S. Sulfur and nitrogen co-doped graphene quantum dots as a fluorescent quenching probe for highly sensitive detection toward mercury ions. ACS Appl. Nano Mater. 2019, 2, 790–798. 10.1021/acsanm.8b02010. [DOI] [Google Scholar]
- Zhang J.; Li Y.; Han S. Simultaneous detection of iodide and mercuric ions by nitrogen-sulfur co-doped graphene quantum dots based on flow injection “turn off-on” chemiluminescence analysis system. Microchem. J. 2019, 147, 1141–1146. 10.1016/j.microc.2019.04.039. [DOI] [Google Scholar]
- Yang Y.; Zou T.; Wang Z.; Xing X.; Peng S.; Zhao R.; Zhang X.; Wang Y. The Fluorescent Quenching Mechanism of N and S Co-Doped Graphene Quantum Dots with Fe3+ and Hg2+ Ions and Their Application as a Novel Fluorescent. Nanomaterials 2019, 9, 738. 10.3390/nano9050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Zhou S.; Xiao L.; Yuan Q.; Gan W. Time-efficient syntheses of nitrogen and sulfur co-doped graphene quantum dots with tunable luminescence and their sensing applications. RSC Adv. 2016, 6, 36554–36560. 10.1039/C6RA05175H. [DOI] [Google Scholar]
- Tian H.; Guo J.; Pang Z.; Hu M.; He J. A sulfur, nitrogen dual-doped porous graphene nanohybrid for ultraselective Hg (ii) separation over Pb (ii) and Cu (ii). Nanoscale 2020, 12, 16543–16555. 10.1039/D0NR04558F. [DOI] [PubMed] [Google Scholar]
- Wang M.; Sun Y.; Yang M. CdS QDs amplified electrochemiluminescence of N, S co-doped graphene quantum dots and its application for Pb (II) determination. Chem. Lett. 2018, 47, 44–47. 10.1246/cl.170846. [DOI] [Google Scholar]
- Liu B.; Ren X.; Chen L.; Ma X.; Chen Q.; Sun Q.; Zhang L.; Si P.; Ci L. High efficient adsorption and storage of iodine on S, N co-doped graphene aerogel. J. Hazard. Mater. 2019, 373, 705–715. 10.1016/j.jhazmat.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Shen C.; Ge S.; Pang Y.; Xi F.; Liu J.; Dong X.; Chen P. Facile and scalable preparation of highly luminescent N, S co-doped graphene quantum dots and their application for parallel detection of multiple metal ions. J. Mater. Chem. B 2017, 5, 6593–6600. 10.1039/C7TB00506G. [DOI] [PubMed] [Google Scholar]
- Li J.; Hou M.; Chen Y.; Cen W.; Chu Y.; Yin S. Enhanced CO2 capture on graphene via N, S dual-doping. Appl. Surf. Sci. 2017, 399, 420–425. 10.1016/j.apsusc.2016.11.157. [DOI] [Google Scholar]
- Kong Q.; Wei C.; Preis S.; Hu Y.; Wang F. Facile preparation of nitrogen and sulfur co-doped graphene-based aerogel for simultaneous removal of Cd2+ and organic dyes. Environ. Sci. Pollut. Res. 2018, 25, 21164–21175. 10.1007/s11356-018-2195-8. [DOI] [PubMed] [Google Scholar]
- Sun P.; Liu H.; Feng M.; Guo L.; Zhai Z.; Fang Y.; Zhang X.; Sharma V. K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Envorin. 2019, 251, 335–345. 10.1016/j.apcatb.2019.03.085. [DOI] [Google Scholar]
- Brindha A.; Sivakumar T. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol., A 2017, 340, 146–156. 10.1016/j.jphotochem.2017.03.010. [DOI] [Google Scholar]
- Ren X.; Feng J.; Si P.; Zhang L.; Lou J.; Ci L. Enhanced heterogeneous activation of peroxydisulfate by S, N co-doped graphene via controlling S, N functionalization for the catalytic decolorization of dyes in water. Chemosphere 2018, 210, 120–128. 10.1016/j.chemosphere.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Quan L.; Qin F. X.; Lu H. T.; Estevez D.; Wang Y. F.; et al. Sequencing dual dopants for an electromagnetic tunable graphene. Chem. Eng. J. 2021, 413, 127421. 10.1016/j.cej.2020.127421. [DOI] [Google Scholar]
- Zhang N.; Chen W.; Chen P.; Wang Y. Insight of S, N co-doped graphene aerogel (double reduction)/cobalt (II)-substituted α-Keggin-type polyoxometalate nanocomposites with synergistically enhanced impedance matching and energy conservation performance. Compos. B. Eng. 2020, 191, 107962. 10.1016/j.compositesb.2020.107962. [DOI] [Google Scholar]
- Sun Z.; Yan Z.; Li A.; Yue K.; Zhao L.; Qian L. Dual heteroatoms co-doping strategy of graphene-based dielectric loss electromagnetic absorbent. Appl. Surf. Sci. 2021, 564, 150380. 10.1016/j.apsusc.2021.150380. [DOI] [Google Scholar]
- Kumar R.; Macedo W. C. Jr; Singh R. K.; Tiwari V. S.; Constantino C. J. L.; Matsuda A.; Moshkalev S. A. Nitrogen–sulfur co-doped reduced graphene oxide-nickel oxide nanoparticle composites for electromagnetic interference shielding. ACS Appl. Nano Mater. 2019, 2 (7), 4626–4636. 10.1021/acsanm.9b01002. [DOI] [Google Scholar]
- Farahmand Habibi M.; Arvand M.; Schröder U.; Sohrabnezhad S. Self-assembled cauliflower-like pyrite-S, N co-doped graphene quantum dots as free-standing anode with high conductivity and biocompatibility for bioelectricity production. Fuel 2021, 286, 119291. 10.1016/j.fuel.2020.119291. [DOI] [Google Scholar]
- Pan M.; Liu S.; Pan B.; Chew J. W. Directionally tailoring the macroscopic polarization of piezocatalysis for hollow zinc sulfide on dual-doped graphene. Nano Energy 2021, 88, 106312. 10.1016/j.nanoen.2021.106312. [DOI] [Google Scholar]
- Pan M.; Liu S.; Chew J. W. Unlocking the high redox activity of MoS2 on dual-doped graphene as a superior piezocatalyst. Nano Energy 2020, 68, 104366. 10.1016/j.nanoen.2019.104366. [DOI] [Google Scholar]
- Wang J.; Ma C.; Mu X.; Zhou X.; He L.; Xiao Y.; Song L.; Hu Y. Designing 3D ternary-structure based on SnO2 nanoparticles anchored hollow polypyrrole microspheres interconnected with N, S co-doped graphene towards high-performance polymer composite. Chem. Eng. J. 2020, 402, 126221. 10.1016/j.cej.2020.126221. [DOI] [Google Scholar]
- Zheng Y.; Jiao Y.; Li L. H.; Xing T.; Chen Y.; Jaroniec M.; Qiao S. Z. Toward Design of Synergistically Active Carbon-Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. 10.1021/nn501434a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.; Wu B.; Liu H.; Tan J.; Hu W.; Liu Y. Direct synthesis of phosphorus and nitrogen co-doped monolayer graphene with air-stable n-type characteristics. Phys. Chem. Chem. Phys. 2014, 16, 20392–20397. 10.1039/C4CP02935F. [DOI] [PubMed] [Google Scholar]
- Li R.; Wei Z.; Gou X. Nitrogen and Phosphorus Dual-Doped Graphene/Carbon Nanosheets as Bifunctional Electrocatalysts for Oxygen Reduction and Evolution. ACS Catal. 2015, 5, 4133–4142. 10.1021/acscatal.5b00601. [DOI] [Google Scholar]
- Ananthanarayanan A.; Wang Y.; Routh P.; Sk M. A.; Than A.; Lin M.; Zhang J.; Chen J.; Sun H.; Chen P. Nitrogen and phosphorus co-doped graphene quantum dots: synthesis from adenosine triphosphate, optical properties, and cellular imaging. Nanoscale 2015, 7, 8159–8165. 10.1039/C5NR01519G. [DOI] [PubMed] [Google Scholar]
- Guo W.; Ma X.; Zhang X.; Zhang Y.; Yu D.; He X. Spinel CoMn2O4 nanoparticles supported on a nitrogen and phosphorus dual doped graphene aerogel as efficient electrocatalysts for the oxygen reduction reaction. RSC Adv. 2016, 6, 96436–96444. 10.1039/C6RA16337H. [DOI] [Google Scholar]
- Chai G.-L.; Qiu K.; Qiao M.; Titirici M.-M.; Shang C.; Guo Z. Active sites engineering leads to exceptional ORR and OER bifunctionality in P, N Co-doped graphene frameworks. Energy Environ. Sci. 2017, 10, 1186–1195. 10.1039/C6EE03446B. [DOI] [Google Scholar]
- Cheng C.; Li Y.; Maouche C.; Li B.; Zhou Y.; Wang S.; Cheng X.; Yang J. Green synthesis of N, P-co doped porous reduced graphene oxide as an active metal-free electrocatalyst toward oxygen reduction reaction. J. Electroanal. Chem. 2021, 883, 115058. 10.1016/j.jelechem.2021.115058. [DOI] [Google Scholar]
- Molina-García M. A.; Rees N. V. Dual-doped graphene/perovskite bifunctional catalysts and the oxygen reduction reaction. Electrochem. Commun. 2017, 84, 65–70. 10.1016/j.elecom.2017.10.004. [DOI] [Google Scholar]
- Liao Y.; Chen H.; Ou C.; Bao L.; Li R.; Liu H. N, P co-doped graphene enriched Phosphorus as a highly efficient oxygen reduction catalyst. J. Electroanal. Chem. 2022, 921, 116560. 10.1016/j.jelechem.2022.116560. [DOI] [Google Scholar]
- Wang J.; Wu Z. X.; Han L. L.; Liu Y. Y.; Guo J. P.; Xin H. L.; Wang D. L. Rational design of three-dimensional nitrogen and phosphorus co-doped graphene nanoribbons/CNTs composite for the oxygen reduction. Chin. Chem. Lett. 2016, 27, 597–601. 10.1016/j.cclet.2016.03.011. [DOI] [Google Scholar]
- Qiao X.; Liao S.; You C.; Chen R. Phosphorus and nitrogen dual doped and simultaneously reduced graphene oxide with high surface area as efficient metal-free electrocatalyst for oxygen reduction. Catalysts 2015, 5, 981–991. 10.3390/catal5020981. [DOI] [Google Scholar]
- Han C.; Chen Z. The mechanism study of oxygen reduction reaction (ORR) on non-equivalent P, N co-doped graphene. Appl. Surf. Sci. 2020, 511, 145382. 10.1016/j.apsusc.2020.145382. [DOI] [Google Scholar]
- Liang Z.; Liu C.; Chen M.; Qi X.; Pramod Kumar U.; Peera S. G.; Liu J.; He J.; Liang T. Oxygen reduction reaction mechanism on P, N co-doped graphene: a density functional theory study. New J. Chem. 2019, 43, 19308–19317. 10.1039/C9NJ04808A. [DOI] [Google Scholar]
- Hung Y. H.; Dutta D.; Tseng C. J.; Chang J. K.; Bhattacharyya A. J.; Su C. Y. Manipulation of heteroatom substitution on nitrogen and phosphorus co-doped graphene as a high active catalyst for hydrogen evolution reaction. J. Phys. Chem. C 2019, 123 (36), 22202–22211. 10.1021/acs.jpcc.9b04607. [DOI] [Google Scholar]
- An L.; Zhang W.; Ma W.; Wang S.; Ma L.; Liu Q.; Guo J.; Zhang X. Ultrafine cobalt–ruthenium alloy on nitrogen and phosphorus co-doped graphene for electrocatalytic water splitting. J. Taiwan Inst. Chem. Eng. 2019, 104, 75–81. 10.1016/j.jtice.2019.08.011. [DOI] [Google Scholar]
- Chen D.; He Z.; Pei S.-E.; Huang L.-A.; Shao H.; Jin Y.; Wang J. Pd nanoparticles supported on N and P dual-doped graphene as an excellent composite catalyst for methanol electro-oxidation. J. Alloys Compd. 2019, 785, 781–788. 10.1016/j.jallcom.2019.01.246. [DOI] [Google Scholar]
- Mahyari M.; Gavgani J. N. Cobalt porphyrin supported on N and P co-doped graphene quantum dots/graphene as an efficient photocatalyst for aerobic oxidation of alcohols under visible-light irradiation. Res. Chem. Intermed. 2018, 44, 3641–3657. 10.1007/s11164-018-3330-3. [DOI] [Google Scholar]
- Xi J.; Wang Q.; Liu J.; Huan L.; He Z.; Qiu Y.; Zhang J.; Tang C.; Xiao J.; Wang S. N, P-dual-doped multilayer graphene as an efficient carbocatalyst for nitroarene reduction: a mechanistic study of metal-free catalysis. J. Catal. 2018, 359, 233–241. 10.1016/j.jcat.2018.01.003. [DOI] [Google Scholar]
- Liang B.; Li K.; Liu Y.; Kang X. Nitrogen and phosphorus dual-doped carbon derived from chitosan: An excellent cathode catalyst in microbial fuel cell. Chem. Eng. J. 2019, 358, 1002–1011. 10.1016/j.cej.2018.09.217. [DOI] [Google Scholar]
- Gong X.; Zhang Q.; Gao Y.; Shuang S.; Choi M. M. F.; Dong C. Phosphorus and nitrogen dual-doped hollow carbon dot as a nanocarrier for doxorubicin delivery and biological imaging. ACS Appl. Mater. Interfaces 2016, 8 (18), 11288–11297. 10.1021/acsami.6b01577. [DOI] [PubMed] [Google Scholar]
- Liu R.; Zhao J.; Huang Z.; Zhang L.; Zou M.; Shi B.; Zhao S. Nitrogen and phosphorus co-doped graphene quantum dots as a nano-sensor for highly sensitive and selective imaging detection of nitrite in live cell. Sens. Actuators B Chem. 2017, 240, 604–612. 10.1016/j.snb.2016.09.008. [DOI] [Google Scholar]
- Shumba M.; Nyokong T. Development of nanocomposites of phosphorus-nitrogen co-doped graphene oxide nanosheets and nanosized cobalt phthalocyanines for electrocatalysis. Electrochim. Acta 2016, 213, 529–539. 10.1016/j.electacta.2016.07.079. [DOI] [Google Scholar]
- Gu X.; Tong C.-J.; Lai C.; Qiu J.; Huang X.; Yang W.; Wen B.; Liu L.-M.; Hou Y.; Zhang S. A porous nitrogen and phosphorous dual doped graphene blocking layer for high performance Li–S batteries. J. Mater. Chem. A 2015, 3, 16670–16678. 10.1039/C5TA04255K. [DOI] [Google Scholar]
- Wu H.; Xia L.; Ren J.; Zheng Q.; Xu C.; Lin D. A high-efficiency N/P co-doped graphene/CNT@ porous carbon hybrid matrix as a cathode host for high performance lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 20458–20472. 10.1039/C7TA06504C. [DOI] [Google Scholar]
- Zhou X.; Liao Q.; Bai T.; Yang J. Rational design of graphene@ nitrogen and phosphorous dual-doped porous carbon sandwich-type layer for advanced lithium–sulfur batteries. J. Mater. Sci. 2017, 52, 7719–7732. 10.1007/s10853-017-1029-2. [DOI] [Google Scholar]
- Zeng P.; Huang L.; Zhang X.; Zhang R.; Wu L.; Chen Y. Long-life and high-areal-capacity lithium-sulfur batteries realized by a honeycomb-like N, P dual-doped carbon modified separator. Chem. Eng. J. 2018, 349, 327–337. 10.1016/j.cej.2018.05.096. [DOI] [Google Scholar]
- Zhang M.; Wang L.; Wang B.; Zhang B.; Sun X.; Wang D.; kong Z.; Xu L. Phosphorus-modified Fe4N@ N, P co-doped graphene as an efficient sulfur host for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 6538–6546. 10.1039/D0TA12361G. [DOI] [Google Scholar]
- Muhammad I.; Jabeen M.; Wang P.; He Y. S.; Liao X. Z.; Ma Z. F. Spray-dried assembly of 3D N, P-Co-doped graphene microspheres embedded with core–shell CoP/MoP@C nanoparticles for enhanced lithium-ion storage. Dalton Trans. 2021, 50, 4555–4566. 10.1039/D1DT00210D. [DOI] [PubMed] [Google Scholar]
- Wang K.; Li Z. Synthesis of nitrogen and phosphorus dual-doped graphene oxide as high-performance anode material for lithium-ion batteries. J. Nanosci. Nanotechnol. 2020, 20, 7673–7679. 10.1166/jnn.2020.18872. [DOI] [PubMed] [Google Scholar]
- Ma X.; Ning G.; Xu C.; Gao J.; Qi C. Phosphorus and Nitrogen Dual-Doped Few-Layered Porous Graphene: A High-Performance Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 14415–14422. 10.1021/am503692g. [DOI] [PubMed] [Google Scholar]
- Li C.; Fu Q.; Zhao K.; Wang Y.; Tang H.; Li H.; Jiang H.; Chen L. Nitrogen and phosphorous dual-doped graphene aerogel with rapid capacitive response for sodium-ion batteries. Carbon 2018, 139, 1117–1125. 10.1016/j.carbon.2018.06.035. [DOI] [Google Scholar]
- Wang Y.; Fu Q.; Li C.; Li H.; Tang H. Nitrogen and Phosphorus Dual-Doped Graphene Aerogel Confined Monodisperse Iron Phosphide Nanodots as an Ultrafast and Long-Term Cycling Anode Material for Sodium-Ion Batteries. ACS Sustainable Chem. Eng. 2018, 6 (11), 15083–15091. 10.1021/acssuschemeng.8b03561. [DOI] [Google Scholar]
- Qin D.; Liu Z.; Zhao Y.; Xu G.; Zhang F.; Zhang X. A sustainable route from corn stalks to N, P-dual doping carbon sheets toward high performance sodium-ion batteries anode. Carbon 2018, 130, 664–671. 10.1016/j.carbon.2018.01.007. [DOI] [Google Scholar]
- Wu Q.; Wang J.; Wang H.; Si Z.; Li C.; Bai J. Doped graphene encapsulated SnP2O7 with enhanced conversion reactions from polyanions as a versatile anode material for sodium dual-ion battery. Electrochim. Acta 2021, 369, 137657. 10.1016/j.electacta.2020.137657. [DOI] [Google Scholar]
- Gao X.; Dong X.; Xing Z.; Nie C.; Zheng G.; Ju Z. Electrolyte Salt Chemistry Enables 3D Nitrogen and Phosphorus Dual-Doped Graphene Aerogels for High-Performance Potassium-Ion Batteries. Adv. Mater. Technol. 2021, 6, 2100207. 10.1002/admt.202100207. [DOI] [Google Scholar]
- Ge L.; Wang D.; Yang P.; Xu H.; Xiao L.; Zhang G. X.; Lu X.; Duan Z.; Meng F.; Zhang J.; An M. Graphite N–C–P dominated three-dimensional nitrogen and phosphorus co-doped holey graphene foams as high-efficiency electrocatalysts for Zn–air batteries. Nanoscale 2019, 11, 17010–17017. 10.1039/C9NR04696H. [DOI] [PubMed] [Google Scholar]
- Shao Q.; Li Y.; Cui X.; Li T.; Wang H.; Li Y.; Duan Q.; Si Z. Metallophthalocyanine-Based Polymer-Derived Co2P Nanoparticles Anchoring on Doped Graphene as High-Efficient Trifunctional Electrocatalyst for Zn-Air Batteries. ACS Sustainable Chem. Eng. 2020, 8 (16), 6422–6432. 10.1021/acssuschemeng.0c00852. [DOI] [Google Scholar]
- Zhang G.; Xing J.; Zhao Y.; Yang F. Hierarchical N, P co-doped graphene aerogels framework assembling vertically grown CoMn-LDH nanosheets as efficient bifunctional electrocatalyst for rechargeable Zinc-air battery. J. Colloid Interface Sci. 2021, 590, 476–486. 10.1016/j.jcis.2021.01.093. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Rufford T. E.; Hulicova-Jurcakova D.; Wang L. Nitrogen and phosphorous co-doped graphene monolith for supercapacitors. ChemSusChem 2016, 9, 513–520. 10.1002/cssc.201501303. [DOI] [PubMed] [Google Scholar]
- Xia K.; Huang Z.; Zheng L.; Han B.; Gao Q.; Zhou C.; Wang H.; Wu J. Facile and controllable synthesis of N/P co-doped graphene for high-performance supercapacitors. J. Power Sources 2017, 365, 380–388. 10.1016/j.jpowsour.2017.09.008. [DOI] [Google Scholar]
- Zhao X.; Wang S.; Wu Q. Nitrogen and phosphorus dual-doped hierarchical porous carbon with excellent supercapacitance performance. Electrochim. Acta 2017, 247, 1140–1146. 10.1016/j.electacta.2017.07.077. [DOI] [Google Scholar]
- Wang C.; Zhao J.; Luo S.; Yu X. Improved Pseudocapacitive Performance of Graphene Architectures Modulating by Nitrogen/Phosphorus Dual-Doping and Steam-Activation. Macromol. Res. 2021, 29, 582–588. 10.1007/s13233-021-9075-7. [DOI] [Google Scholar]
- Wei F.; Wei Y.; Wang J.; Han M.; Lv Y. N, P dual doped foamy-like carbons with abundant defect sites for zinc ion hybrid capacitors. Chem. Eng. J. 2022, 450, 137919. 10.1016/j.cej.2022.137919. [DOI] [Google Scholar]
- Cheng H.; Yi F.; Gao A.; Liang H.; Shu D.; Zhou X.; He C.; Zhu Z. Supermolecule self-assembly promoted porous N, P Co-doped reduced graphene oxide for high energy density supercapacitors. ACS Appl. Energy Mater. 2019, 2 (6), 4084–4091. 10.1021/acsaem.9b00204. [DOI] [Google Scholar]
- Li G.; Li Y.; Deng J.; Lin H.; Hou X.; Jia L. Ultrahigh rate capability supercapacitors based on tremella-like nitrogen and phosphorus co-doped graphene. Mater. Chem. Front. 2020, 4, 2704–2715. 10.1039/D0QM00392A. [DOI] [Google Scholar]
- Sandhiya M.; Sathish M. Enhanced electrochemical performance of supercritical fluid treated N, P co-doped graphene by dual redox-additives. J. Power Sources 2022, 540, 231587. 10.1016/j.jpowsour.2022.231587. [DOI] [Google Scholar]
- Zhao Y.; Hao H.; Song T.; Wang X.; Li C.; Li W. High energy-power density Zn-ion hybrid supercapacitors with N/P co-doped graphene cathode. J. Power Sources 2022, 521, 230941. 10.1016/j.jpowsour.2021.230941. [DOI] [Google Scholar]
- Nazarian-Samani M.; Haghighat-Shishavan S.; Nazarian-Samani M.; Kim M.-S.; Cho B.-W.; Oh S.-H.; Kashani-Bozorg S. F.; Kim K.-B. Rational hybrid modulation of P, N dual-doped holey graphene for high-performance supercapacitors. J. Power Sources 2017, 372, 286–296. 10.1016/j.jpowsour.2017.10.087. [DOI] [Google Scholar]
- Wang C.; Luo S.; Yang Y.; Ren D.; Yu X. Defect-Rich Graphene Architecture Induced by Nitrogen and Phosphorus Dual Doping for High-Performance Supercapacitors. Energy Technology 2020, 8, 1900685. 10.1002/ente.201900685. [DOI] [Google Scholar]
- Zhang M.; Liu H.; Ma T.; Song Z.; Shao S. Ultrathin porous Mn(PO3)2 nanosheets and MoO2 nanocrystal arrays on N, P-dual-doped graphene for high-energy asymmetric supercapacitor. Chem. Eng. J. 2021, 403, 126379. 10.1016/j.cej.2020.126379. [DOI] [Google Scholar]
- Wang Z.; Tan Y.; Yang Y.; Zhao X.; Liu Y.; Niu L.; et al. Pomelo peels-derived porous activated carbon microsheets dual-doped with nitrogen and phosphorus for high performance electrochemical capacitors. J. Power Sources 2018, 378, 499–510. 10.1016/j.jpowsour.2017.12.076. [DOI] [Google Scholar]
- Li H.; Liu T.; He Y.; Song J.; Meng A.; Sun C.; Hu M.; Wang L.; Li G.; Zhang Z.; Liu Y.; Zhao J.; Li Z. Interfacial Engineering and a Low-Crystalline Strategy for High-Performance Supercapacitor Negative Electrodes: Fe2P2O7 Nanoplates Anchored on N/P Co-doped Graphene Nanotubes. ACS Appl. Mater. Interfaces 2022, 14 (2), 3363–3373. 10.1021/acsami.1c17356. [DOI] [PubMed] [Google Scholar]
- Deng Y.; Wang H.; Zhang K.; Qiu J.; Yan L. Flexible Quasi-Solid-State High-Performance Aqueous Zinc Ion Hybrid Supercapacitor with Water-in-Salt Hydrogel Electrolyte and N/P-Dual Doped Graphene. Adv. Sustainable Syst. 2022, 6, 2100191. 10.1002/adsu.202100191. [DOI] [Google Scholar]
- Rahimi-Aghdam T.; Shariatinia Z.; Hakkarainen M.; Haddadi-Asl V. Nitrogen and phosphorous doped graphene quantum dots: Excellent flame retardants and smoke suppressants for polyacrylonitrile nanocomposites. J. Hazard. Mater. 2020, 381, 121013. 10.1016/j.jhazmat.2019.121013. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Wang B.; Li X.; Ye Y.; Ma J.; Liu C.; Zhou X.; Xie X. Enhancing thermal oxidation and fire resistance of reduced graphene oxide by phosphorus and nitrogen co-doping: Mechanism and kinetic analysis. Carbon 2019, 146, 650–659. 10.1016/j.carbon.2019.01.099. [DOI] [Google Scholar]
- Feng Y.; He C.; Wen Y.; Ye Y.; Zhou X.; Xie X.; Mai Y. W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J. Hazard. Mater. 2018, 346, 140–151. 10.1016/j.jhazmat.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Esrafili M. D.; Mohammad-Valipour R.; Mousavi-Khoshdel S. M.; Nematollahi P. A Comparative Study of CO Oxidation on Nitrogen-and Phosphorus-Doped Graphene. ChemPhysChem 2015, 16, 3719–3727. 10.1002/cphc.201500488. [DOI] [PubMed] [Google Scholar]
- Han C.; Chen Z. Adsorption properties of O2 on the unequal amounts of binary co-doped graphene by B/N and P/N: a density functional theory study. Appl. Surf. Sci. 2019, 471, 445–454. 10.1016/j.apsusc.2018.12.019. [DOI] [Google Scholar]
- Cruz-Silva E.; López-Urías F.; Muñoz-Sandoval E.; Sumpter B. G.; Terrones H.; Charlier J. C.; Meunier V.; Terrones M. Electronic Transport and Mechanical Properties of Phosphorus and Phosphorus–Nitrogen-Doped Carbon Nanotubes. ACS Nano 2009, 3, 1913–1921. 10.1021/nn900286h. [DOI] [PubMed] [Google Scholar]
- Yuan B.; Xing W.; Hu Y.; Mu X.; Wang J.; Tai Q.; Li G.; Liu L.; Liew K. M.; Hu Y. Boron/phosphorus doping for retarding the oxidation of reduced graphene oxide. Carbon 2016, 101, 152–158. 10.1016/j.carbon.2016.01.080. [DOI] [Google Scholar]
- Meng J.; Tong Z.; Sun H.; Liu Y.; Zeng S.; Xu J.; Xia Q.; Pan Q.; Dou S.; Yu H. Metal-Free Boron/Phosphorus Co-Doped Nanoporous Carbon for Highly Efficient Benzyl Alcohol Oxidation. Adv. Sci. 2022, 9, 2200518. 10.1002/advs.202200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G.; Huang K.; Ma J.-S.; Ju Z.; Xing Z.; Zhuang Q.-C. Phosphorus and oxygen dual-doped graphene as superior anode material for room-temperature potassium-ion batteries. J. Mater. Chem. A 2017, 5, 7854–7861. 10.1039/C7TA01108C. [DOI] [Google Scholar]
- Yu X.; Kang Y.; Park H. S. Sulfur and phosphorus co-doping of hierarchically porous graphene aerogels for enhancing supercapacitor performance. Carbon 2016, 101, 49–56. 10.1016/j.carbon.2016.01.073. [DOI] [Google Scholar]
- Patel M. A.; Luo F.; Savaram K.; Kucheryavy P.; Xie Q.; Flach C.; Mendelsohn F.; Garfunkel E.; Lockard J. V.; He H. P and S dual-doped graphitic porous carbon for aerobic oxidation reactions: Enhanced catalytic activity and catalytic sites. Carbon 2017, 114, 383–392. 10.1016/j.carbon.2016.11.064. [DOI] [Google Scholar]
- Liu H.; Zhang M.; Ma T.; Wang Y. Ni and NiO in situ grown on sulfur and phosphorus co-doped graphene as effective bifunctional catalyst for hydrogen evolution. J. Electroanal. Chem. 2019, 848, 113306. 10.1016/j.jelechem.2019.113306. [DOI] [Google Scholar]
- An M.; Du L.; Du C.; Sun Y.; Wang Y.; Yin G.; Gao Y. Pt nanoparticles supported by sulfur and phosphorus co-doped graphene as highly active catalyst for acidic methanol electrooxidation. Electrochim. Acta 2018, 285, 202–213. 10.1016/j.electacta.2018.07.237. [DOI] [Google Scholar]
- Jing C.; Guo X.; Xia L.; Chen Y.; Wang X.; Liu X.; Dong B.; Dong F.; Li S.; Zhang Y. Morphologically confined hybridization of tiny CoNi2S4 nanosheets into S, P co-doped graphene leading to enhanced pseudocapacitance and rate capability. Chem. Eng. J. 2020, 379, 122305. 10.1016/j.cej.2019.122305. [DOI] [Google Scholar]
- Zhang M.; Wang Y.; Liu H.; Ma T.; Xie J.; Shao S. Controllable synthesis of CoNb2O6 nanoparticles on multifunctional sulfur and phosphorus dual-doped graphene as advanced electrodes for hybrid supercapacitors. Electrochim. Acta 2019, 309, 104–115. 10.1016/j.electacta.2019.04.021. [DOI] [Google Scholar]
- Yu X.; Pei C.; Feng L. Surface modulated hierarchical graphene film via sulfur and phosphorus dual-doping for high performance flexible supercapacitors. Chin. Chem. Lett. 2019, 30, 1121–1125. 10.1016/j.cclet.2019.01.009. [DOI] [Google Scholar]
- Chen X.; Li H.; Zhang G.; Feng S.; Yang G.; Shi L.; Liu W.; Liu G.; Pan H. Au@Pt Hybrid Nanorods Encapsulated in B, S dual-doped Graphene as Highly Sensitive Immunosensing Platform for Electrochemical Determination of Aflatoxin B1. Int. J. Electrochem. Sci. 2020, 15, 6269–6289. 10.20964/2020.07.19. [DOI] [Google Scholar]
- Olaniyan O.; Mapasha R. E.; Momodu D. Y.; Madito M. J.; et al. Exploring the stability and electronic structure of beryllium and sulphur co-doped graphene: a first principles study. RSC Adv. 2016, 6, 88392–88402. 10.1039/C6RA17640B. [DOI] [Google Scholar]
- Niu F.; Liu J. M.; Tao L. M.; Wang W.; Song W. G. Nitrogen and silica co-doped graphene nanosheets for NO2 gas sensing. J. Mater. Chem. A 2013, 1, 6130–6133. 10.1039/c3ta11070b. [DOI] [Google Scholar]
- Zhou X.; Zhao C.; Wu G.; Chen J.; Li Y. DFT study on the electronic structure and optical properties of N, Al, and N-Al doped graphene. Appl. Surf. Sci. 2018, 459, 354–362. 10.1016/j.apsusc.2018.08.015. [DOI] [Google Scholar]
- Li S.; Lu Z.; Zhang Y.; Ma D.; Yang Z. Mechanisms of direct hydrogen peroxide synthesis on silicon and phosphorus dual-doped graphene: a DFT-D study. Phys. Chem. Chem. Phys. 2017, 19, 9007–9015. 10.1039/C6CP08668C. [DOI] [PubMed] [Google Scholar]
- Liu H.; Tang Y.; Zhao W.; Ding W.; Xu J.; Liang C.; Zhang Z.; Huang F. Facile Synthesis of Nitrogen and Halogen Dual-Doped Porous Graphene as an Advanced Performance Anode for Lithium-Ion Batteries. Adv. Mater. Interfaces 2018, 5, 1701261. 10.1002/admi.201701261. [DOI] [Google Scholar]
- Denis P. A. Mono and dual doped monolayer graphene with aluminum, silicon, phosphorus and sulfur. Comp. Theor Chem. 2016, 1097, 40–47. 10.1016/j.comptc.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Ullah S.; Denis P. A.; Sato F. First-principles study of dual-doped graphene: towards promising anode materials for Li/Na-ion batteries. New J. Chem. 2018, 42, 10842–10851. 10.1039/C8NJ01098F. [DOI] [Google Scholar]
- Ullah S.; Denis P. A.; Sato F. Adsorption and diffusion of alkali-atoms (Li, Na, and K) on BeN dual doped graphene. Int. J. Quantum Chem. 2019, 119, e25900. 10.1002/qua.25900. [DOI] [Google Scholar]
- Hussain A.; Ullah S.; Farhan M. A. Fine tuning the band-gap of graphene by atomic and molecular doping: a density functional theory study. RSC Adv. 2016, 6, 55990–56003. 10.1039/C6RA04782C. [DOI] [Google Scholar]
- Olaniyan O.; Maphasha R. E.; Madito M. J.; Khaleed A. A.; Igumbor E.; Manyala N. A systematic study of the stability, electronic and optical properties of beryllium and nitrogen co-doped graphene. Carbon 2018, 129, 207–227. 10.1016/j.carbon.2017.12.014. [DOI] [Google Scholar]
- Denis P. A.; Iribarne F. Dual doped monolayer and bilayer graphene: The case of 4p and 2p elements. Chem. Phys. Lett. 2016, 658, 152. 10.1016/j.cplett.2016.06.032. [DOI] [PubMed] [Google Scholar]
- Safaei Ardakani Y. S.; Moradi M. A DFT/TDDFT study on dual doped bilayer graphene containing Se and X (Ga, P, S). Eur. Phys. J. B 2020, 93, 99. 10.1140/epjb/e2020-100579-0. [DOI] [Google Scholar]
- Chen J.; Lin C.; Zhang M.; Jin T.; Qian Y. Constructing Nitrogen, Selenium Co-Doped Graphene Aerogel Electrode Materials for Synergistically Enhanced Capacitive Performance. ChemElectroChem. 2020, 7, 3311. 10.1002/celc.202000635. [DOI] [Google Scholar]
- Zhong Y.; Xu H.; Zhang Q.; Song X.; Hao C. The Structural Design of Dual-Element-Doped Graphene for Iodine Reduction Reaction: Density Functional Theory Study. ChemistrySelect 2022, 7, e202200380. 10.1002/slct.202200380. [DOI] [Google Scholar]
- Dong Q.; Zhuang X.; Li Z.; Li B.; Fang B.; Yang C.; Xie H.; Zhang F.; Feng X. Efficient approach to iron/nitrogen co-doped graphene materials as efficient electrochemical catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 7767–7772. 10.1039/C5TA00556F. [DOI] [Google Scholar]
- Zitolo A.; Goellner V.; Armel V.; Sougrati M. T.; Mineva T.; Stievano L.; Fonda E.; Jaouen F. Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. 10.1038/nmat4367. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Huang X.; Hu W. Fe3C nanoparticle decorated Fe/N doped graphene for efficient oxygen reduction reaction electrocatalysis. J. Power Sources 2016, 332, 305–311. 10.1016/j.jpowsour.2016.09.130. [DOI] [Google Scholar]
- Jiang H.; Yao Y.; Zhu Y.; Liu Y.; Su Y.; Yang X.; Li C. Iron carbide nanoparticles encapsulated in mesoporous Fe–N-doped graphene-like carbon hybrids as efficient bifunctional oxygen electrocatalysts. ACS Appl. Mater. Interfaces 2015, 7 (38), 21511–21520. 10.1021/acsami.5b06708. [DOI] [PubMed] [Google Scholar]
- Sibul R.; Kibena-Põldsepp E.; Ratso S.; Kook M.; Sougrati M. T.; Käärik M.; Merisalu M.; Aruväli J.; Paiste P.; Treshchalov A.; Leis J.; Kisand V.; Sammelselg V.; Holdcroft S.; Jaouen F.; Tammeveski K. Iron-and Nitrogen-Doped Graphene-Based Catalysts for Fuel Cell Applications. ChemElectroChem. 2020, 7, 1739–1747. 10.1002/celc.202000011. [DOI] [Google Scholar]
- Zhang Z.; Huang X.; Xu H. Anchoring an Fe Dimer on Nitrogen-Doped Graphene toward Highly Efficient Electrocatalytic Ammonia Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 43632–43640. 10.1021/acsami.1c11585. [DOI] [PubMed] [Google Scholar]
- Gao X. X.; Zhou X.; Ma Y. F.; Wang C. P.; Chu F. A fluorometric and colorimetric dual-mode sensor based on nitrogen and iron co-doped graphene quantum dots for detection of ferric ions in biological fluids and cellular imaging. New J. Chem. 2018, 42, 14751–14756. 10.1039/C8NJ01805G. [DOI] [Google Scholar]
- Zhang L.; Liang P.; Man X.; Wang D.; Huang J.; Shu H.; Liu Z.; Wang L. Fe, N co-doped graphene as a multi-functional anchor material for lithium-sulfur battery. J. Phys. Chem. Solids. 2019, 126, 280–286. 10.1016/j.jpcs.2018.11.027. [DOI] [Google Scholar]
- Zeng Q. W.; Hu R. M.; Chen Z. B.; Shang J. X. Single-atom Fe and N co-doped graphene for lithium-sulfur batteries: a density functional theory study. Mater. Res. Express 2019, 6, 095620 10.1088/2053-1591/ab33ad. [DOI] [Google Scholar]
- Ni Y.; Chen Z.; Kong F.; Qiao Y.; Kong A.; Shan Y. Space-confined synthesis of multilayer Cu–N-doped graphene nanosheets for efficient oxygen electroreduction. Dalton Trans. 2017, 46, 8586–8592. 10.1039/C7DT01614J. [DOI] [PubMed] [Google Scholar]
- Zhu G.; Liu F.; Wang Y.; Wei Z.; Wang W. Systematic exploration of N, C coordination effects on the ORR performance of Mn–Nx doped graphene catalysts based on DFT calculations. Phys. Chem. Chem. Phys. 2019, 21, 12826–12836. 10.1039/C9CP02155H. [DOI] [PubMed] [Google Scholar]
- Luo M.; Liang Z.; Liu C.; Qi X.; Chen M.; Sagar R. U. R.; Yang H.; Liang T. Single–atom manganese and nitrogen co-doped graphene as low-cost catalysts for the efficient CO oxidation at room temperature. Appl. Surf. Sci. 2021, 536, 147809. 10.1016/j.apsusc.2020.147809. [DOI] [Google Scholar]
- Lee B. H.; Hasan M. T.; Lichthardt D.; Gonzalez-Rodriguez R.; Naumov A. V. Manganese–nitrogen and gadolinium–nitrogen Co-doped graphene quantum dots as bimodal magnetic resonance and fluorescence imaging nanoprobes. Nanotechnology 2021, 32, 095103 10.1088/1361-6528/abc642. [DOI] [PubMed] [Google Scholar]
- Li F.; Shu H.; Hu C.; Shi Z.; Liu X.; Liang P.; Chen X. Atomic mechanism of electrocatalytically active Co–N complexes in graphene basal plane for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7 (49), 27405–27413. 10.1021/acsami.5b09169. [DOI] [PubMed] [Google Scholar]
- Han M.; Shi M.; Wang J.; Zhang M.; Yan C.; Jiang J.; Guo S.; Sun Z.; Guo Z. Efficient bifunctional Co/N dual-doped carbon electrocatalysts for oxygen reduction and evolution reaction. Carbon 2019, 153, 575–584. 10.1016/j.carbon.2019.07.075. [DOI] [Google Scholar]
- Liu X.; Amiinu I. S.; Liu S.; Cheng K.; Mu S. Transition metal/nitrogen dual-doped mesoporous graphene-like carbon nanosheets for the oxygen reduction and evolution reactions. Nanoscale 2016, 8, 13311–13320. 10.1039/C6NR03247H. [DOI] [PubMed] [Google Scholar]
- Du Z.; Chen X.; Hu W.; Chuang C.; Xie S.; Hu A.; Yan W.; Kong X.; Wu X.; Ji H.; Wan L. J. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium–sulfur batteries. J. Am. Chem. Soc. 2019, 141 (9), 3977–3985. 10.1021/jacs.8b12973. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Liang P.; Shu H. B.; Man X. L.; Du X. Q.; Chao C. L.; Liu Z. G.; Sun Y. P.; Wan H. Z.; Wang W. Design rules of heteroatom-doped graphene to achieve high performance lithium–sulfur batteries: both strong anchoring and catalysing based on first principles. J. Colloid Interface Sci. 2018, 529, 426–431. 10.1016/j.jcis.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Xiao M.; Zhu J.; Li G.; Li N.; Li S.; Cano Z. P.; Ma L.; Cui P.; Xu P.; Jiang G.; Jin H.; Wang S.; Wu T.; Lu J.; Yu A.; Su D.; Chen Z. Single atom iridium heterogeneous catalyst in oxygen reduction reaction. Angew. Chem., Int. Ed. 2019, 58, 9640. 10.1002/anie.201905241. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Zhang M.; Zhu B.; Gao Y. Investigation of the Stability and Hydrogen Evolution Activity of Dual-Atom Catalysts on Nitrogen-Doped Graphene. Nanomaterials 2022, 12, 2557. 10.3390/nano12152557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P.; Feng H.; Wang S.; Ding H.; Liang Y.; Ling M.; Zhang X. Electrocatalytic CO2 reduction reaction on dual-metal- and nitrogen-doped graphene: coordination environment effect of active sites. Mater. Adv. 2022, 3, 4566–4577. 10.1039/D2MA00192F. [DOI] [Google Scholar]
- Kharangarh P. R.; Gupta V.; Singh A.; Bhardwaj P.; Grace A. N. An efficient pseudocapacitor electrode material with co-doping of iron (II) and sulfur in luminescent graphene quantum dots. Diam. Relat. Mater. 2020, 107, 107913. 10.1016/j.diamond.2020.107913. [DOI] [Google Scholar]
- Gu J.; Zhang X.; Fu L.; Pang A. Study on the hydrogen storage properties of the dual active metals Ni and Al doped graphene composites. Int. J. Hydrogen Energy 2019, 44, 6036–6044. 10.1016/j.ijhydene.2019.01.057. [DOI] [Google Scholar]
- Ullah S.; Denis P. A.; Sato F. Triple-Doped Monolayer Graphene with Boron, Nitrogen, Aluminum, Silicon, Phosphorus, and Sulfur. ChemPhysChem 2017, 18, 1864–1873. 10.1002/cphc.201700278. [DOI] [PubMed] [Google Scholar]
- Razmjooei F.; Singh K. P.; Song M. Y.; Yu J. S. Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: a comprehensive study. Carbon 2014, 78, 257–267. 10.1016/j.carbon.2014.07.002. [DOI] [Google Scholar]
- Wang W.; Wang X.; Xing J.; Gong Q.; Wang H.; Wang J.; Chen Z.; Ai Y.; Wang X. Multi-heteroatom doped graphene-like carbon nanospheres with 3D inverse opal structure: A promising bisphenol-A remediation material. Environ. Sci.: Nano 2019, 6, 809–819. 10.1039/C8EN01196F. [DOI] [Google Scholar]
- Wang Y.; Zhang B.; Xu M.; He X. Tunable ternary (P, S, N)-doped graphene as an efficient electrocatalyst for oxygen reduction reaction in an alkaline medium. RSC Adv. 2015, 5, 86746–86753. 10.1039/C5RA18251D. [DOI] [Google Scholar]
- Dou S.; Shen A.; Ma Z.; Wu J.; Tao L.; Wang S. N-, P-and S-tridoped graphene as metal-free electrocatalyst for oxygen reduction reaction. J. Electroanal. Chem. 2015, 753, 21–27. 10.1016/j.jelechem.2015.05.013. [DOI] [Google Scholar]
- Wang Y.; Xu N.; He R.; Peng L.; Cai D.; Qiao J. Large-scale defect-engineering tailored tri-doped graphene as a metal-free bifunctional catalyst for superior electrocatalytic oxygen reaction in rechargeable Zn-air. Appl. Catal., B 2021, 285, 119811. 10.1016/j.apcatb.2020.119811. [DOI] [Google Scholar]
- Zheng X.; Wu J.; Cao X.; Abbott J.; Jin C.; Wang H.; Strasser P.; Yang R.; Chen X.; Wu G. N-, P-, and S-doped graphene-like carbon catalysts derived from onium salts with enhanced oxygen chemisorption for Zn-air battery cathodes. Appl. Catal. B: Environ. 2019, 241, 442–451. 10.1016/j.apcatb.2018.09.054. [DOI] [Google Scholar]
- Xu Y.; Wang S.; Hou X.; Sun Z.; Jiang Y.; Dong Z.; Tao Q.; Man J.; Cao Y. Coal-derived nitrogen, phosphorus and sulfur co-doped graphene quantum dots: A promising ion fluorescent probe. Appl. Surf. Sci. 2018, 445, 519–526. 10.1016/j.apsusc.2018.03.156. [DOI] [Google Scholar]
- Li Y.; Wen H.; Yang J.; Zhou Y.; Cheng X. Boosting oxygen reduction catalysis with N, F, and S tri-doped porous graphene: tertiary N-precursors regulates the constitution of catalytic active sites. Carbon 2019, 142, 1–12. 10.1016/j.carbon.2018.09.079. [DOI] [Google Scholar]
- Murugesan B.; Pandiyan N.; Arumugam M.; et al. Two dimensional graphene oxides converted to three dimensional P, N, F and B, N, F tri-doped graphene by ionic liquid for efficient catalytic performance. Carbon 2019, 151, 53–67. 10.1016/j.carbon.2019.05.060. [DOI] [Google Scholar]
- Kundu S.; Sarojinijeeva P.; Karthick R.; Anantharaj G.; Saritha G.; Bera R.; Anandan S.; Patra A.; Ragupathy P.; Selvaraj M.; Jeyakumar D.; Pillai K. V. Enhancing the Efficiency of DSSCs by the Modification of TiO2 Photoanodes using N, F and S, co-doped Graphene Quantum Dots. Electrochim. Acta 2017, 242, 337–343. 10.1016/j.electacta.2017.05.024. [DOI] [Google Scholar]
- Kundu S.; Yadav R. M.; Narayanan T. N.; Shelke M. V.; Vajtai R.; Ajayan P. M.; Pillai V. K. Synthesis of N, F and S co-doped graphene quantum dots. Nanoscale 2015, 7, 11515–11519. 10.1039/C5NR02427G. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Dai L. Nitrogen, phosphorus, and fluorine tri-doped graphene as a multifunctional catalyst for self-powered electrochemical water splitting. Angew. Chem., Int. Ed. 2016, 55, 13296. 10.1002/anie.201607405. [DOI] [PubMed] [Google Scholar]
- Lin H.; Chu L.; Wang X.; Yao Z.; Liu F.; Ai Y.; Zhuang X.; Han S. Boron, nitrogen, and phosphorous ternary doped graphene aerogel with hierarchically porous structures as highly efficient electrocatalysts for oxygen reduction reaction. New J. Chem. 2016, 40, 6022–6029. 10.1039/C5NJ03390J. [DOI] [Google Scholar]
- Dong F.; Cai Y.; Liu C.; Liu J.; Qiao J. Heteroatom (B, N and P) doped porous graphene foams for efficient oxygen reduction reaction electrocatalysis. Int. J. Hydrog. Energy 2018, 43, 12661–12670. 10.1016/j.ijhydene.2018.04.118. [DOI] [Google Scholar]
- Zhao Y.; Huang S.; Xia M.; Rehman S.; Mu S.; Kou Z.; et al. N-P-O co-doped high performance 3D graphene prepared through red phosphorous-assisted “cutting-thin” technique: A universal synthesis and multifunctional. Nano Energy 2016, 28, 346–355. 10.1016/j.nanoen.2016.08.053. [DOI] [Google Scholar]
- Hu R.; Li Y.; Zeng Q.; Shang J. Role of active sites in N-coordinated Fe-Co dual-metal doped graphene for oxygen reduction and evolution reactions: A theoretical insight. Appl. Surf. Sci. 2020, 525, 146588. 10.1016/j.apsusc.2020.146588. [DOI] [Google Scholar]
- He T.; Santiago A. R. P.; Du A. Atomically embedded asymmetrical dual-metal dimers on N-doped graphene for ultra-efficient nitrogen reduction reaction. J. Catal. 2020, 388, 77–83. 10.1016/j.jcat.2020.05.009. [DOI] [Google Scholar]
- Zhou Y.; Yang W.; Utetiwabo W.; Lian Y.; Yin X.; Zhou L.; Yu P.; Chen R.; Sun S. Revealing of active sites and catalytic mechanism in N-coordinated Fe, Ni dual-doped carbon with superior acidic oxygen reduction than single-atom catalyst. J. Phys. Chem. Lett. 2020, 11 (4), 1404–1410. 10.1021/acs.jpclett.9b03771. [DOI] [PubMed] [Google Scholar]
- Wu C.; Yang W.; Wang J. J.; Li H.; Gates I. D. Methane activation on dual-atom catalysts supported on graphene. Chem. Commun. 2021, 57, 12127–12130. 10.1039/D1CC05701D. [DOI] [PubMed] [Google Scholar]
- Qiao X.; You C.; Shu T.; Fu Z.; Zheng R.; Zeng X.; Li X.; Liao S. A one-pot method to synthesize high performance multielement co-doped reduced graphene oxide catalysts for oxygen reduction. Electrochem. Commun. 2014, 47, 49–53. 10.1016/j.elecom.2014.07.024. [DOI] [Google Scholar]
- Feng M.; Zhang Q.; Sun S.; Zhang X.; Hu S. In-situ synthesis of Fe, N, S co-doped graphene-like nanosheets around carbon nanoparticles with dual-nitrogen-source as efficient electrocatalyst for oxygen reduction reaction. Int. J. Hydrog. Energy 2021, 46, 8002–8013. 10.1016/j.ijhydene.2020.12.036. [DOI] [Google Scholar]
- Molina-García M. A.; Rees N. V. “Metal-free” electrocatalysis: Quaternary-doped graphene and the alkaline oxygen reduction reaction. Appl. Catal., A 2018, 553, 107–116. 10.1016/j.apcata.2017.12.014. [DOI] [Google Scholar]
- Nguyen G. D.; Toma F. M.; Cao T.; Pedramrazi Z.; Chen C.; Rizzo D. J.; Joshi T.; Bronner C.; Chen Y.-C.; Favaro M.; Louie S. G.; Fischer F. R.; Crommie M. F. Crommie, Bottom-Up Synthesis of N = 13 Sulfur-Doped Graphene Nanoribbons. J. Phys. Chem. C 2016, 120 (5), 2684–2687. 10.1021/acs.jpcc.5b09986. [DOI] [Google Scholar]




